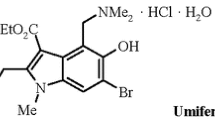
We report new carboanalogs of umifenovir – regioisomeric derivatives of ethyl 5-hydroxy-(trans-2-phenylcyclopropyl)-1Н-indole-3-carboxylate. The inhibition of HIV replication by umifenovir and its carboanalogs at micromolar concentration range has been demonstrated for the first time.


Similar content being viewed by others
Notes
The unambiguous assignment is not possible due to the proximity of the signals for protons H-6 and H-7 of the indole moiety.
References
Balzarini, J.; Ruchko, E. A.; Zakharova, E. K.; Kameneva, I. Yu.; Nawrozkij, M. B. Chem. Heterocycl. Compd. 2014, 50, 489. [Khim. Geterotsikl. Soedin. 2014, 50, 537.]
Blaising, J.; Polyak, S. J.; Pécheur, E.-I. Antivir. Res. 2014, 107, 84.
Sheridan, R. P. J. Chem. Inf. Comput. Sci. 2002, 42, 103.
Mai, А.; Artico, M.; Rotili, D.; Tarantino, D.; Clotet-Codina, I.; Armand-Ugón, M.; Ragno, R.; Simeoni, S.; Sbardella, G.; Nawrozkij, M. B.; Samuele, A.; Maga, G.; Esté, J. A. J. Med. Chem. 2007, 50, 5412.
Rotili, D.; Samuele, A.; Tarantino, D.; Ragno, R.; Musmuca, I.; Ballante, F.; Botta, G.; Morera, L.; Pierini, M.; Cirilli, R.; Nawrozkij, M. B.; Gonzalez, E.; Clotet, B.; Artico, M.; Esté, J. A.; Maga, G.; Mai, А. J. Med. Chem. 2012, 55, 3558.
Grinshtein, V. Ya.; Anderson, M. Ya. Izv. Akad. Nauk Latv. SSR, Ser. Khim. 1963, 1, 106.
Organicum [Russian translation]; Reutov, O. A., Ed.; Mir: Moscow, 1992, Vol. 2, p. 69.
Bell, M. R.; Oesterlin, R.; Beyler, A. L.; Harding, H. R.; Potts, G. O. J. Med. Chem. 1967, 10, 264.
Kucklander, U. Arch. Pharm. 1971, 304, 602.
Kucklander, U. Tetrahedron 1972, 28, 5251.
Grinev, A. N.; Kul'bovskaya, N. K.; Terent'ev, A. P. Zh. Obshch. Khim. 1955, 25, 1355.
Grinev, A. N.; Yermakova, V. N.; Terent'ev, A. P. Zh. Obshch. Khim. 1962, 32, 1948.
Allen, G. R., Jr. Org. React. 1973, 20, 385.
Pawlak, J. M.; Khau, V. V.; Hutchison, D. R.; Martinelli, M. J. J. Org. Chem. 1996, 61, 9055.
Romero, D. L.; Morge, R. A.; Genin, M. J.; Biles, C.; Busso, M.; Resnick, L.; Althaus, I. W.; Reusser, F.; Thomas, R. C.; Tarpley, W. G. J. Med. Chem. 1993, 36, 1505.
Jiang, T.; Kuhen, K. L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Wu, T. Y.-H.; He, Y. Bioorg. Med. Chem. Lett. 2006, 16, 2105.
Jiang, T.; Kuhen, K. L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Tuntland, T.; Zhang, K.; Karanewsky, D.; He, Y. Bioorg. Med. Chem. Lett. 2006, 16, 2109.
Tietze, L. F.; Eicher, T. Preparative Organic Chemistry. Reactions and Syntheses in the Organic Chemistry Laboratory [Russian translation]; Alekseeva, Yu. E., Ed.; Mir: Moscow, 1999.
Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyter, J.; De Clercq, E. J. Virol. Methods 1988, 20, 309.
This work received financial support from the Russian Foundation for Basic Research (grants NK 13-03-00144/14 and MD-1658.2014.3, for the preparation and study of compound rac-МС-1501).
The authors would like to acknowledge the contribution by graduate student E. K. Zakharova at the Organic Chemistry Department of Volgograd State Technical University in isolation and purification of the target compounds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(11/12), 978–983
* For Communication 1, see 1.
Rights and permissions
About this article
Cite this article
Schols, D., Ruchko, E.A., Lavrenov, S.N. et al. Structural analogs of umifenovir 2*. The synthesis and antiHIV activity study of new regioisomeric (trans-2-phenylcyclopropyl)-1Н-indole derivatives. Chem Heterocycl Comp 51, 978–983 (2015). https://doi.org/10.1007/s10593-016-1807-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1807-9