Abstract
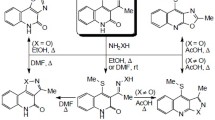
Methods have been developed for the synthesis of 2,3′-biquinolines, their 1′,4′-dihydro derivatives, 3-pyrid-2-ylquinolines, and 3-pyrazin-2-ylquinoline based on the interaction of hetarylethylenes and vinyl butyl ether with imidoyl chlorides and Vilsmeier complexes. Using the example of the synthesis of 3-hetarylquinolines and their dihydro derivatives the synthetic possibilities of the Vilsmeier method were shown for creating various bonds in different quinoline nuclei of the bisheterocyclic system.
Similar content being viewed by others
References
T. P. Glushchenko, V. I. Goncharov, and A. V. Aksenov, Khim. Geterotsikl. Soedin., 409 (2008). [Chem. Heterocycl. Comp., 44, 313 (2008)].
H. Weidel, Monatsh., 2, 491 (1881).
A. V. Aksenov, I. V. Magedov, and Yu. I. Smushkevich, J. Chem. Soc., Perkin Trans. 1, 759 (1992).
A. V. Aksenov, A. Yu. Polykarpov, Yu. I. Smushkevich, and I. V. Magedov, J. Chem. Res. (S), 402 (1994).
M. Ishikura, I. Oda, and M. Terashima, Heterocycles, 2375 (1985).
E. Carlier and A. Einhorn, Ber., 23, 2894 (1890).
W. Borsche and R. Manteuffel, Liebigs Ann. Chem., 526, 22 (1936).
W. H. Mills and H. G. Orgish, J. Chem. Soc., 81 (1928).
G. Koller and H. Ruppersberg, Monatsh., 58, 238 (1931).
F. Krőhnke, H. Dickhäuser, and I. Vogt, Liebigs Ann. Chem., 644, 93 (1961).
V. V. Trifonov, I. V. Aksenova, V. I. Goncharov, and A. V. Aksenov, Khim. Geterotsikl. Soedin., 1867 (2005). [Chem. Heterocycl. Comp., 41, 1541 (2005)].
A. V. Aksenov, O. N. Nadein, I. V. Borovlev, and Yu. I. Smushkevich, Khim. Geterotsikl. Soedin., 350 (1998). [Chem. Heterocycl. Comp., 34, 316 (1998)].
A. V. Aksenov and N. V. Demidova, Khim. Geterotsikl. Soedin., 1051 (2002). [Chem. Heterocycl. Comp., 38, 913 (2002)].
S. Dumouchel, F. Mongin, F. Trecourt, and G. Queguiner, Tetrahedron, 59, 8629 (2003).
J. Mathieu, P. Gros, and Y. Fort, Tetrahedron Lett., 41, 1879 (2001).
D. V. Moiseev and A. V. Aksenov, Khim. Geterotsikl. Soedin., 707 (2001). [Chem. Heterocycl. Comp., 37, 654 (2001)].
A. V. Aksenov, I. V. Aksenova, I. V. Borovlev, and Yu. I. Smushkevich, Khim. Geterotsikl. Soedin., 1094 (1997). [Chem. Heterocycl. Comp., 33, 954 (1997)].
A. V. Aksenov, O. N. Nadein, I. V. Borovlev, and Yu. I. Smushkevich, Khim. Geterotsikl. Soedin., 232 (1998). [Chem. Heterocycl. Comp., 34, 207 (1998)].
A. V. Aksenov, D. V. Moiseev, I. V. Borovlev, and O. N. Nadein, Khim. Geterotsikl. Soedin., 1084 (2000). [Chem. Heterocycl. Comp., 36, 948 (2000)].
A. V. Aksenov, O. N. Nadein, D. V. Moiseev, and Yu. I. Smushkevich, Khim. Geterotsikl. Soedin., 919 (1999). [Chem. Heterocycl. Comp., 35, 804 (1999)].
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1209–1215, August, 2008.
Rights and permissions
About this article
Cite this article
Glushenko, T.P., Goncharov, V.I. & Aksenov, A.V. Investigations in 2,3′-biquinoline series 24. Synthesis of 3-hetarylquinolines and their 1,4-dihydro derivatives under conditions of the Vilsmeier reaction. Chem Heterocycl Comp 44, 973–978 (2008). https://doi.org/10.1007/s10593-008-0138-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-008-0138-x