Abstract
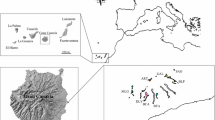
Analysis of levels and patterns of genetic variation in a rare species is important for determining whether genetic factors associated with small population size, such as genetic drift or inbreeding, may be negatively affecting a species. In this study, we compared estimates of genetic diversity and patterns of population genetic structure in a rare cliff endemic, Erigeron lemmonii, to those of a widespread congener, E. arisolius. Our goals were to assess whether rarity and small population size have negatively affected levels of genetic diversity in E. lemmonii and to identify genetic threats that may limit the ability of E. lemmonii to persist. Levels of observed and expected heterozygosity and allelic richness in E. lemmonii were approximately 60 % of those found in E. arisolius. After correcting for null alleles, inbreeding coefficients in both species of Erigeron were very small, suggesting that both species are highly outcrossing and may demonstrate self-incompatibility. Patterns of genetic structure in both species revealed almost no population substructuring, indicating that widespread gene flow is occurring within each species. Because we found no evidence for inbreeding or a genetic bottleneck in E. lemmonii, it is likely that the species’ lower genetic diversity may be the result of genetic drift. Because E. lemmoni exists in a single population, no other populations exist to bolster population size or genetic diversity in the event of declines; thus, conservation efforts should focus on seed collection from as many individuals as possible to protect against possible future losses of genetic diversity. We also recommend continued monitoring of both population size and genetic diversity in E. lemmonii to ensure the species’ long-term persistence and viability.

Similar content being viewed by others
References
(58 FR 51144) Endangered and threatened wildlife and plants; Review of plant taxa for listing and endangered or threatened species. 58 Federal Register 205 (25 Oct 1999), p 51144–51190
(77 FR 60509) Endangered and threatened wildlife and plants; 12-Month finding for the lemmon fleabane; Endangered status for the Acuña Cactus and the Fickeisen plains cactus and designation of critical habitat. 77 Federal Register 192 (3 October 2012), p 60509–60579
Allen AM, Thorogood CJ, Hegarty MJ, Lexer C, Hiscock SJ (2011) Pollen-pistil interactions and self-incompatibility in the Asteraceae: new insights from studies of Senecio squalidus (Oxford ragwort). Ann Bot 108:687–698
Allphin L, Wiens D, Harper KT (2002) The relative effects of resources and genetics on reproductive success in the rare Kachina daisy, Erigeron kachinensis (Asteraceae). Int J Plant Sci 163:599–612
Armbruster WS, McGuire AD (1991) Experimental assessment of reproductive interactions between sympatric Aster and Erigeron (Asteraceae) in Interior Alaska. Am J Bot 78:1449–1457
Bailey P (2013) Pollination biology of the endemic Erigeron lemmonii A. Gray, and its insect visitor networks compared to two widespread congeners Erigeron arisolius G.L. Nesom and Erigeron neomexicanus A. Gray (Asteraceae) PhD dissertation, Department of Environmental Biology. University of Guelph, Guelph, Ontario, Canada
Byers DL, Meagher TR (1992) Mate availability in small populations of plant species with homomorphic sporophytic self-incompatibility. Heredity 68:353–359
Chapuis MP, Estoup A (2007) Microsatellite null alleles and estimation of population differentiation. Mol Biol Evol 24:621–631
Chybicki IJ, Burczyk J (2009) Simultaneous estimation of null alleles and inbreeding coefficients. J Hered 100:106–113
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Cronquist A (1947) Revision of the North American species of Erigeron. Brittonia 6:121–300
DeSalle R, Amato G (2004) The expansion of conservation genetics. Nat Rev Genet 5:702–712
Dieringer D, Schlötterer C (2003) Microsatellite analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169
Earl D, vonHoldt B (2012) Structure harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res 4:359–361
El Mousadik A, Petit R (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839
Ellis JR, Pashley CH, Burke JM, McCauley DE (2006) High genetic diversity in a rare and endangered sunflower as compared to a common congener. Mol Ecol 15:2345–2355
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Ferrer MM, Good-Avila SV (2007) Macrophylogenetic analyses of the gain and loss of self-incompatibility in the Asteraceae. New Phytol 173:401–414
Fischer M, Hock M, Paschke M (2003) Low genetic variation reduces cross-compatibility and offspring fitness in populations of a narrow endemic plant with a self-incompatibility system. Conserv Genet 4:325–336
Frankham R (1995) Conservation genetics. Annu Rev Genet 29:305–327
Frankham R, Ralls K (1998) Inbreeding leads to extinction. Nature 392:441–442
Gitzendanner MA, Soltis PS (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87:783–792
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Gray A (1883) Contributions to North American botany. Proc Am Acad Arts 19:1–96
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos T Roy Soc B 351:1291–1298
Hartl DL, Clark AG (1989) Principles of Population Genetics. Sinauer Associates Inc., Sunderland
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Karron JD (1987) A comparison of levels of genetic polymorphism and self-compatibility in geographically restricted and widespread plant congeners. Evol Ecol 1:47–58
Lindsay DL, Bailey P, Anderson JL, Jung MG, Edwards CE, Lance RF (2012) Isolation and characterization of microsatellite loci for a hyper-rare cliff endemic, Erigeron lemmonii, and a more widespread congener, Erigeron arisolius (Asteraceae). Conserv Genet Res 4:849–852
Luikart G, Cornuet JM (1998) Empirical evaluation of a test for identifying recently bottlenecked populations from allele frequency data. Conserv Biol 12:228–237
Luikart G, Allendorf FW, Cornuet JM, Sherwin WB (1998) Distortion of allele frequency distributions provides a test for recent population bottlenecks. J Hered 89:238–247
Malusa J (2006) Surveys and monitoring plots of the Lemmon’s fleabane, Erigeron lemmonii, in the Huachuca Mts, Arizona. A report to the United States Fish and Wildlife Service, the University of Arizona and the Arizona Department of Agriculture
Meirmans PG, Van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Nesom G (1989a) Infrageneric taxonomy of New World Erigeron. Phytologia 67:67–93
Nesom GL (1989b) A new species of Erigeron (Asteraceae:Astereae) from Arizona. Phytologia 67(4):304–306
Nesom GL (1990) Taxonomy of the Erigeron coronarius group of Erigeron sect. Geniculatis (Asteraceae: Astereae). Phytologia 69:237–253
Nesom G (2006) Erigeron lemmonii. In: Committee FNA (ed) Flora of North America North of Mexico, vol 16. Oxford, New York, p 342
Noyes RD (2000) Biogeographical and evolutionary insights on Erigeron and allies (Asteraceae) from ITS sequence data. Plant Syst Evol 220:93–114
Noyes R, Bailey P (2013) Chromosome number and reproductive attributes for Erigeron lemmonii (Asteraceae), a cliff-dwelling endemic of southeastern Arizona. Madroño In press
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Piry S, Luikart G, Cornuet JM (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:502–503
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Ranker TA (1994) Evolution of high genetic variability in the rare Hawaiian fern Adenophorus periens and implications for conservation management. Biol Conserv 70:19–24
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rousset F (2008) GENEPOP ‘007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Res 8:103–106
Schierup MH, Vekemans X (2008) Genomic consequences of selection on self-incompatibility genes. Curr Opin Plant Biol 11:116–122
Willi Y, Van Buskirk J, Fischer M (2005) A threefold genetic Allee effect: population size affects cross-compatibility, inbreeding depression and drift load in the self-incompatible Ranunculus reptans. Genetics 169:2255–2265
Acknowledgments
The authors thank M. G. Jung, J. L. Anderson, C. G. Cox, B. L. Escalon, and A. J. Hollingsworth for assistance with field and lab work, S. Stone for assistance with collection permits and access to Fort Huachuca; and H. L. Farrington and S. Stone for comments on a previous version of this manuscript. The study described and the resulting data presented herein were obtained from research conducted under a 6.1 Basic Research grant (Project 09-03) from the U.S. Army Engineer Research and Development Center’s Environmental Quality Technology Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Christine E. Edwards and Denise L. Lindsay contributed equally to this study
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Edwards, C.E., Lindsay, D.L., Bailey, P. et al. Patterns of genetic diversity in the rare Erigeron lemmoni and comparison with its more widespread congener, Erigeron arisolius (Asteraceae). Conserv Genet 15, 419–428 (2014). https://doi.org/10.1007/s10592-013-0549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-013-0549-9