Abstract
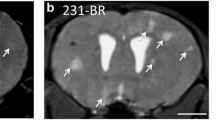
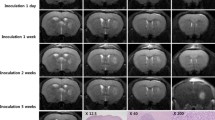
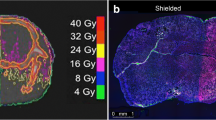
To facilitate the development of new brain metastasis (BM) treatment, an easy-to-use and clinically relevant animal model with imaging platform is needed. Rhabdomyosarcoma BM was induced in WAG/Rij rats. Post-implantation surveillance and characterizations were systematically performed with multiparametric MRI including 3D T1 and T2 weighted imaging, diffusion-weighted imaging (DWI), T1 and T2 mapping, and perfusion-weighted imaging (PWI), which were validated by postmortem digital radiography (DR), µCT angiography and histopathology. The translational potential was exemplified by the application of a vascular disrupting agent (VDA). BM was successfully induced in most rats of both genders (18/20). Multiparametric MRI revealed significantly higher T2 value, pre-contrast-enhanced (preCE) T1 value, DWI-derived apparent diffusion coefficient (ADC) and CE ratio, but a lower post-contrast-enhanced (postCE) T1 value in BM lesions than in adjacent brain (p < 0.01). PWI showed the dynamic and higher contrast agent uptake in the BM compared with the adjacent brain. DR, µCT and histopathology characterized the BM as hypervascular tumors. After VDA treatment, the BM showed drug-related perfusion changes and partial necrosis as evidenced by anatomical, functional MRI parameters and postmortem findings. The present BM model and imaging modalities represent a feasible and translational platform for developing BM-targeting therapeutics.







Similar content being viewed by others
Data availability
All data in this study is available under reasonable request.
Code availability
Python codes are available under reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- BBB:
-
Blood–brain barrier
- BBTB:
-
Blood–brain-tumor-barrier
- BM:
-
Brain metastasis
- CA:
-
Contrast agent
- CA4P:
-
Combretastatin A4 phosphate
- CE:
-
Contrast-enhanced
- DKI:
-
Diffusion kurtosis imaging
- DR:
-
Digital radiography
- DWI:
-
Diffusion-weighted imaging
- IHC:
-
Immunohistochemistry
- µCT:
-
Micro computed tomography
- MRI:
-
Magnetic resonance imaging
- postCE:
-
Post-contrast-enhanced
- preCE:
-
Pre-contrast-enhanced
- PDX:
-
Patient-derived xenograft
- PWI:
-
Dynamic contrast enhanced perfusion weighted imaging
- R1:
-
Rhabdomyosarcoma
- SPACE:
-
Sampling perfection with application optimized contrasts using different flip angle evolution
- SI:
-
Signal intensity
- TSE:
-
Turbo spin echo
- VDA:
-
Vascular-disrupting agent
References
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics. CA A Cancer J Clin 70(1):7–30
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331:1559–1564
Cagney DN et al (2017) Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19(11):1511–1521
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1):48–54
Brower JV et al (2016) Management of leptomeningeal metastases: Prognostic factors and associated outcomes. J Clin Neurosci 27:130–137
Suh JH et al (2020) Current approaches to the management of brain metastases. Nat Rev Clin Oncol 17(5):279–299
Valiente M et al (2018) The Evolving Landscape of Brain Metastasis. Trends Cancer 4(3):176–196
Duchnowska R et al (2012) Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res 14(4):R119
Shah N et al (2020) Drug resistance occurred in a newly characterized preclinical model of lung cancer brain metastasis. BMC Cancer 20(1):292
Teleanu RI et al (2019) Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med 9(1):84
Vredenburgh JJ et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729
Liu Y et al (2018) The first study on therapeutic efficacies of a vascular disrupting agent CA4P among primary hepatocellular carcinomas with a full spectrum of differentiation and vascularity: Correlation of MRI-microangiography-histopathology in rats. Int J Cancer 143(7):1817–1828
Shi C et al (2017) Monitoring Tumor response to antivascular therapy using non-contrast intravoxel incoherent motion diffusion-weighted MRI. Can Res 77(13):3491
Li J et al (2011) A dual-targeting anticancer approach: soil and seed principle. Radiology 260(3):799–807
Li J et al (2013) Sequential systemic administrations of combretastatin A4 Phosphate and radioiodinated hypericin exert synergistic targeted theranostic effects with prolonged survival on SCID mice carrying bifocal tumor xenografts. Theranostics 3(2):127–137
Abma E et al (2019) Power Doppler ultrasound and contrast-enhanced ultrasound demonstrate non-invasive tumour vascular response to anti-vascular therapy in canine cancer patients. Sci Rep 9(1):9262
Genovesi LA et al (2021) Patient-derived orthotopic xenograft models of medulloblastoma lack a functional blood-brain barrier. Neuro Oncol 23(5):732–742
Arvanitis CD, Ferraro GB, Jain RK (2020) The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer 20(1):26–41
Heye AK et al (2014) Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review Neuroimage Clin 6:262–274
Imaging CfAIiM Public database of AIMI.
Yushkevich PA et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128
Yin T et al (2017) Characterization of a rat orthotopic pancreatic head tumor model using three-dimensional and quantitative multi-parametric MRI. NMR Biomed 30(2):3676
McGrath DM et al (2009) Comparison of model-based arterial input functions for dynamic contrast-enhanced MRI in tumor bearing rats. Magn Reson Med 61(5):1173–1184
Team RC (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Do J et al (2014) Ex vivo Evans blue assessment of the blood brain barrier in three breast cancer brain metastasis models. Breast Cancer Res Treat 144(1):93–101
Yao L et al (2018) Evans Blue Dye: A Revisit of Its Applications in Biomedicine. Contrast Media Mol Imaging 2018:7628037
Ireson CR et al (2019) The role of mouse tumour models in the discovery and development of anticancer drugs. British J Cancer 121(2):101–108
Brighi C et al (2020) Comparative study of preclinical mouse models of high-grade glioma for nanomedicine research: the importance of reproducing blood-brain barrier heterogeneity. Theranostics 10(14):6361–6371
Olson B et al (2018) Mouse models for cancer immunotherapy research. Cancer Discov 8(11):1358–1365
Fink KR, Fink JR (2013) Imaging of brain metastases. Surg Neurol Int 4(4):S209–S219
Castets CR et al (2016) In vivo MEMRI characterization of brain metastases using a 3D Look-Locker T1-mapping sequence. Sci Rep 6(1):39449
Bontempi P et al (2021) Quantitative multicomponent T2 relaxation showed greater sensitivity than flair imaging to detect subtle alterations at the periphery of lower grade gliomas. Front Oncol 11:834
Chou MC et al (2009) Correlation between the MR T2 value at 47 T and relative water content in articular cartilage in experimental osteoarthritis induced by ACL transection. Osteoarthritis Cartilage 17(4):441–447
Ordovas KG, Higgins CB (2011) Delayed contrast enhancement on MR images of myocardium: past, present Future. Radiology 261(2):358–374
Yin T et al (2017) Vascular disrupting agent in pancreatic and hepatic tumour allografts: observations of location-dependent efficacy by MRI, microangiography and histomorphology. Br J Cancer 117(10):1529–1536
Schoen S Jr et al (2021) Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv Drug Deliv Rev 180:114043
Puchalapalli M et al (2016) NSG mice provide a better spontaneous model of breast cancer metastasis than athymic (Nude) Mice. PLoS ONE 11(9):e0163521
Acknowledgements
We thank Prof. Stefaan Soenen from Nanohealth and Optical Imaging Group in KU Leuven for providing rhabdomyosarcoma cell line. We also appreciate the Center for Artificial Intelligence in Medicine & Imaging at Stanford University for making the clinical brain MRI images publicly available with special thanks to Prof. Dr. Greg Zaharchuk from department of radiology, Stanford University for approving the use of clinical brain metastasis images in figure 2. We sincerely thank Ahmed Radwan from department of imaging and pathology, KU Leuven and Sjoerd Nooijens from department of cardiovascular sciences, KU Leuven, Belgium, who actively participated in the discussion of imaging analyses. We acknowledge Oncocidia Limited, London, UK and Mr. Jean-Pierre Peters with his firm P&R Medical, Hasselt, Belgium for their partial financial supports to our ongoing research.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
SW Performing experiment, statistical analyses and drafting manuscript; LC Performing experiment, and statistical analyses; YF MRI image segementation, drafting manuscript and discussion; TY MRI image analyses, and discussion; JY Pathological analyses, and discussion; FDK MRI image analyses, and discussion; RP MRI image analyses, and discussion; CVO MRI image analyses, and discussion; GB Supervision, and discussion; JS Supervision, and discussion; JS Performing experiments, uCT image analyses, discussion; MW Performing experiments, uCT image analyses, discussion; YL Study design, reviewing manuscript; YN Supervision of study, study design, reviewing manuscript, MRI image segementation.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
This study was approved by the ethical committee of KU Leuven with a registry No. P046/2019 and executed with laboratory animal care.
Consent to participate
No applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 10240 kb)
Supplementary file3 (MP4 10317 kb)
Supplementary file4 (MP4 11035 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Chen, L., Feng, Y. et al. Development and characterization of a rat brain metastatic tumor model by multiparametric magnetic resonance imaging and histomorphology. Clin Exp Metastasis 39, 479–493 (2022). https://doi.org/10.1007/s10585-022-10155-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-022-10155-w