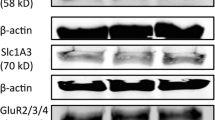
Abstract
Bone is one of the most frequent sites for metastasis of breast and prostate cancers. Bone metastases are associated with pathologic changes in bone turnover and severe pain. The mechanisms that trigger these effects are not well understood, but it is postulated that tumour cells release factors which interfere with signalling processes critical to bone homeostasis. We have identified that several cancer cell lines known to cause bone disruption in animal models of bone metastasis appear to secrete glutamate into their extracellular environment in vitro. Although these cells also express specific glutamate receptors, the implications of this potentially disruptive chemical signal are discussed in relation to normal glutamate-dependent communication processes in bone and a possible mechanistic connection is made between tumour cell glutamate release and the development of pathological changes in bone turnover.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Abbreviations
- CNS:
-
Central nervous system
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- EDTA:
-
Ethylenediaminetetraacetic acid (chelating agent)
- MEM:
-
Minimal essential medium
- NMDA:
-
N-methyl-d-aspartate
- NMDAR:
-
N-methyl-d-aspartate receptor (NMDA type glutamate receptor)
- RPMI-1640:
-
Roswell Park Memorial Institute 1640 medium
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
References
Coleman RE (1997) Skeletal complications of malignancy. Cancer 80(8, Suppl):1588–1594. doi:10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G
Orr FW, Lee J, Duivenvoorden WC, Singh G (2000) Pathophysiologic interactions in skeletal metastasis. Cancer 88(12, Suppl):2912–2918. doi:10.1002/1097-0142(20000615)88:12+<2912::AID-CNCR6>3.0.CO;2-8
Vukmirovic-Popovic S, Colterjohn N, Lhotak S, Duivenvoorden WC, Orr FW, Singh G (2002) Morphological, histomorphometric, and microstructural alterations in human bone metastasis from breast carcinoma. Bone 31(4):529–535. doi:10.1016/S8756-3282(02)00847-5
Rose AA, Siegel PM (2006) Breast cancer-derived factors facilitate osteolytic bone metastasis. Bull Cancer 93(9):931–943
Bonfil RD, Chinni S, Fridman R, Kim HR, Cher ML (2007) Proteases, growth factors, chemokines, and the microenvironment in prostate cancer bone metastasis. Urol Oncol 25(5):407–411. doi:10.1016/j.urolonc.2007.05.008
Skerry TM (2008) The role of glutamate in the regulation of bone mass and architecture. J Musculoskelet Neuronal Interact 8(2):166–173
Bussard KM, Gay CV, Mastro AM (2008) The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev 27(1):41–55. doi:10.1007/s10555-007-9109-4
Chenu C (2002) Glutamatergic regulation of bone remodeling. J Musculoskelet Neuronal Interact 2(3):282–284
Hinoi E, Takarada T, Yoneda Y (2004) Glutamate signaling system in bone. J Pharmacol Sci 94(3):215–220. doi:10.1254/jphs.94.215
Esquenazi S, Monnerie H, Kaplan P, Le Roux P (2002) BMP-7 and excess glutamate: opposing effects on dendrite growth from cerebral cortical neurons in vitro. Exp Neurol 176(1):41–54. doi:10.1006/exnr.2002.7906
Li T, Ghishan FK, Bai L (2005) Molecular physiology of vesicular glutamate transporters in the digestive system. World J Gastroenterol 11(12):1731–1736
Skerry TM, Genever PG (2001) Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci 22(4):174–181. doi:10.1016/S0165-6147(00)01642-4
Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y (2004) Glutamate signaling in peripheral tissues. Eur J Biochem 271(1):1–13. doi:10.1046/j.1432-1033.2003.03907.x
Serre CM, Farlay D, Delmas PD, Chenu C (1999) Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25(6):623–629. doi:10.1016/S8756-3282(99)00215-X
Chenu C (2002) Glutamatergic regulation of bone resorption. J Musculoskelet Neuronal Interact 2(5):423–431
Chenu C (2002) Glutamatergic innervation in bone. Microsc Res Tech 58(2):70–76. doi:10.1002/jemt.10120
Spencer GJ, Genever PG (2003) Long-term potentiation in bone—a role for glutamate in strain-induced cellular memory? BMC Cell Biol 4:9. doi:10.1186/1471-2121-4-9
Szczesniak AM, Gilbert RW, Mukhida M, Anderson GI (2005) Mechanical loading modulates glutamate receptor subunit expression in bone. Bone 37(1):63–73. doi:10.1016/j.bone.2003.10.016
Takarada T, Yoneda Y (2008) Pharmacological topics of bone metabolism: glutamate as a signal mediator in bone. J Pharmacol Sci 106(4):536–541. doi:10.1254/jphs.FM0070243
Patton AJ, Genever PG, Birch MA, Suva LJ, Skerry TM (1998) Expression of an N-methyl-D-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone 22(6):645–649. doi:10.1016/S8756-3282(98)00061-1
Chenu C, Serre CM, Raynal C, Burt-Pichat B, Delmas PD (1998) Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone 22(4):295–299. doi:10.1016/S8756-3282(97)00295-0
Laketic-Ljubojevic I, Suva LJ, Maathuis FJ, Sanders D, Skerry TM (1999) Functional characterization of N-methyl-D-aspartic acid-gated channels in bone cells. Bone 25(6):631–637. doi:10.1016/S8756-3282(99)00224-0
Gu Y, Genever PG, Skerry TM, Publicover SJ (2002) The NMDA type glutamate receptors expressed by primary rat osteoblasts have the same electrophysiological characteristics as neuronal receptors. Calcif Tissue Int 70(3):194–203. doi:10.1007/s00223-001-2004-z
Hinoi E, Fujimori S, Takemori A, Kurabayashi H, Nakamura Y, Yoneda Y (2002) Demonstration of expression of mRNA for particular AMPA and kainate receptor subunits in immature and mature cultured rat calvarial osteoblasts. Brain Res 943(1):112–116. doi:10.1016/S0006-8993(02)02726-9
Mason DJ, Suva LJ, Genever PG, Patton AJ, Steuckle S, Hillam RA et al (1997) Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone 20(3):199–205. doi:10.1016/S8756-3282(96)00386-9
Stains JP, Civitelli R (2005) Cell-to-cell interactions in bone. Biochem Biophys Res Commun 328(3):721–727. doi:10.1016/j.bbrc.2004.11.078
Kato S, Negishi K, Mawatari K, Kuo CH (1992) A mechanism for glutamate toxicity in the C6 glioma cells involving inhibition of cystine uptake leading to glutathione depletion. Neuroscience 48(4):903–914. doi:10.1016/0306-4522(92)90278-A
Ye ZC, Sontheimer H (1999) Glioma cells release excitotoxic concentrations of glutamate. Cancer Res 59(17):4383–4391
Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK et al (2001) Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta 1512(2):335–344. doi:10.1016/S0005-2736(01)00338-8
Sontheimer H (2003) Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci 26(10):543–549. doi:10.1016/j.tins.2003.08.007
Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY et al (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25(31):7101–7110. doi:10.1523/JNEUROSCI.5258-04.2005
Duivenvoorden WC, Popovic SV, Lhotak S, Seidlitz E, Hirte HW, Tozer RG et al (2002) Doxycycline decreases tumor burden in a bone metastasis model of human breast cancer. Cancer Res 62(6):1588–1591
Duivenvoorden WC, Vukmirovic-Popovic S, Kalina M, Seidlitz E, Singh G (2007) Effect of zoledronic acid on the doxycycline-induced decrease in tumour burden in a bone metastasis model of human breast cancer. Br J Cancer 96:1526–1531. doi:10.1038/sj.bjc.6603740
Hinoi E, Fujimori S, Yoneda Y (2003) Modulation of cellular differentiation by N-methyl-D-aspartate receptors in osteoblasts. FASEB J 17(11):1532–1534
Merle B, Itzstein C, Delmas PD, Chenu C (2003) NMDA glutamate receptors are expressed by osteoclast precursors and involved in the regulation of osteoclastogenesis. J Cell Biochem 90(2):424–436. doi:10.1002/jcb.10625
Morimoto R, Uehara S, Yatsushiro S, Juge N, Hua Z, Senoh S et al (2006) Secretion of L-glutamate from osteoclasts through transcytosis. EMBO J 25(18):4175–4186. doi:10.1038/sj.emboj.7601317
Taylor AF (2002) Functional osteoblastic ionotropic glutamate receptors are a prerequisite for bone formation. J Musculoskelet Neuronal Interact 2(5):415–422
Bhangu PS, Genever PG, Spencer GJ, Grewal TS, Skerry TM (2001) Evidence for targeted vesicular glutamate exocytosis in osteoblasts. Bone 29(1):16–23. doi:10.1016/S8756-3282(01)00482-3
Bhangu PS (2003) ‘Pre-synaptic’ vesicular glutamate release mechanisms in osteoblasts. J Musculoskelet Neuronal Interact 3(1):17–29
Genever PG, Skerry TM (2001) Regulation of spontaneous glutamate release activity in osteoblastic cells and its role in differentiation and survival: evidence for intrinsic glutamatergic signaling in bone. FASEB J 15(9):1586–1588
Kalariti N, Lembessis P, Papageorgiou E, Pissimissis N, Koutsilieris M (2007) Regulation of the mGluR5, EAAT1 and GS expression by glucocorticoids in MG-63 osteoblast-like osteosarcoma cells. J Musculoskelet Neuronal Interact 7(2):113–118
Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H (2007) Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 67(19):9463–9471. doi:10.1158/0008-5472.CAN-07-2034
Wu Y, Shen D, Chen Z, Clayton S, Vadgama JV (2007) Taxol induced apoptosis regulates amino acid transport in breast cancer cells. Apoptosis 12(3):593–612. doi:10.1007/s10495-006-0007-y
Franklin RB, Zou J, Yu Z, Costello LC (2006) EAAC1 is expressed in rat and human prostate epithelial cells; functions as a high-affinity L-aspartate transporter; and is regulated by prolactin and testosterone. BMC Biochem 7:10. doi:10.1186/1471-2091-7-10
Carrascosa JM, Martinez P, Nunez de Castro I (1984) Nitrogen movement between host and tumor in mice inoculated with Ehrlich ascitic tumor cells. Cancer Res 44(9):3831–3835
Collins CL, Wasa M, Souba WW, Abcouwer SF (1998) Determinants of glutamine dependence and utilization by normal and tumor-derived breast cell lines. J Cell Physiol 176(1):166–178. doi:10.1002/(SICI)1097-4652(199807)176:1<166::AID-JCP18>3.0.CO;2-5
Gonzalez-Cadavid NF, Ryndin I, Vernet D, Magee TR, Rajfer J (2000) Presence of NMDA receptor subunits in the male lower urogenital tract. J Androl 21(4):566–578
Maxwell M, McCoy TA, Neuman RE (1956) The amino acid requirements of the Walker carcinosarcoma 256 in vitro. Cancer Res 16(10 Part 1):979–984
Abdul M, Hoosein N (2005) N-methyl-D-aspartate receptor in human prostate cancer. J Membr Biol 205(3):125–128. doi:10.1007/s00232-005-0777-0
Rzeski W, Turski L, Ikonomidou C (2001) Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA 98(11):6372–6377. doi:10.1073/pnas.091113598
Rzeski W, Ikonomidou C, Turski L (2002) Glutamate antagonists limit tumor growth. Biochem Pharmacol 64(8):1195–1200. doi:10.1016/S0006-2952(02)01218-2
Acknowledgments
This work was supported by operating grants from the Canadian Institutes of Health Research to G.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seidlitz, E.P., Sharma, M.K., Saikali, Z. et al. Cancer cell lines release glutamate into the extracellular environment. Clin Exp Metastasis 26, 781–787 (2009). https://doi.org/10.1007/s10585-009-9277-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-009-9277-4