Abstract
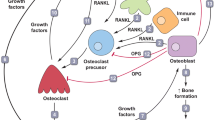
Bone metastases cause severe skeletal morbidity including fractures and hypercalcemia. Tumor cells in bone induce activation of osteoclasts, which mediate bone resorption and release of growth factors from bone matrix, resulting in a “vicious cycle” of bone breakdown and tumor proliferation. Receptor activator of NF-κB ligand (RANKL) is an essential mediator of osteoclast formation, function, and survival, and is blocked by a soluble decoy receptor, osteoprotegerin (OPG). In human malignancies that metastasize to bone, dysregulation of the RANK/RANKL/OPG pathway can increase the RANKL:OPG ratio, a condition which favors excessive osteolysis. In a mouse model of bone metastasis, RANKL protein levels in MDA-MB-231 (MDA-231) tumor-bearing bones were significantly higher than tumor-free bones. The resulting tumor-induced osteoclastogenesis and osteolysis was dose-dependently inhibited by recombinant OPG-Fc treatment, supporting the essential role for RANKL in this process. Using bioluminescence imaging in a mouse model of metastasis, we monitored the anti-tumor efficacy of RANKL inhibition on MDA-231 human breast cancer cells in a temporal manner. Treatment with OPG-Fc in vivo inhibited growth of MDA-231 tumor cells in bony sites when given both as a preventative (dosed day 0) and as a therapeutic agent for established bone metastases (dosed day 7). One mechanism by which RANKL inhibition reduced tumor burden appears to be indirect through inhibition of the “vicious cycle” and involved an increase in tumor cell apoptosis, as measured by active caspase-3. Here, we demonstrate for the first time that OPG-Fc treatment of mice with established bone metastases resulted in an overall improvement in survival.







Similar content being viewed by others
Abbreviations
- BLI:
-
Bioluminescence imaging
- MDA-231Luc:
-
Luciferase-labeled MDA-231 cells
- OPG:
-
Osteoprotegerin
- OPG-Fc:
-
Human osteoprotegerin: Fc domain fusion protein
- RANK:
-
Receptor activator of NF-κB
- RANKL:
-
Receptor activator of NF-κB ligand
References
Coleman RE (1997) Skeletal complications of malignancy. Cancer 80:1588–1594
Taube T, Elomaa I, Blomqvist C et al (1994) Histomorphometric evidence for osteoclast-mediated bone resorption in metastatic breast cancer. Bone 15:161–166
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Fuller K, Wong B, Fox S et al (1998) TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 188:997–1001
Kong YY, Yoshida H, Sarosi I et al (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Lacey DL, Tan HL, Lu J et al (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157:435–448
Lacey DL, Timms E, Tan HL et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Bucay N, Sarosi I, Dunstan CR et al (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268
Simonet WS, Lacey DL, Dunstan CR et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350:1655–1664
Terpos E, Szydlo R, Apperley JF et al (2003) Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102:1064–1069
Mountzios G, Dimopolous M-A, Bamias A et al (2006) Abnormal bone remodeling process is due to an imbalance in the receptor activator of nuclear factor/kB ligand (RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors metastatic to the skeleton. Acta Oncol 46:221–229
Morony S, Capparelli C, Sarosi I et al (2001) Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 61:4432–4436
Miller R, Jones J, Tometsko M et al (2005) Antitumor efficacy of the RANK ligand inhibitor OPG-Fc in the MDA-231 breast cancer and PC3 prostate cancer experimental osteolytic metastases models. J Bone Miner Res 20:S117
Zheng Y, Zhou H, Brennan K et al (2007) Inhibition of bone resorption, rather than direct cytotoxicity, mediates the anti-tumour actions of ibandronate and osteoprotegerin in a murine model of breast cancer bone metastasis. Bone 40:471–478
Morony S, Sarosi I, Doerr N et al (2000) OPG prevents bone destruction and decreases skeletal tumor burden in an experimental model of tumor metastasis to bone. J Bone Miner Res 15:S209
Dougall WC, Chaisson M (2006) The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev 25:541–549
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Sasaki A, Boyce BF, Story B et al (1995) Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res 55:3551–3557
Dull T, Zufferey R, Kelly M et al (1998) A third-generation lentivirus vector with a conditional packaging system. J Virol 72:8463–8471
Morony S, Capparelli C, Lee R et al (1999) A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1beta, TNF-alpha, PTH, PTHrP, and 1,25(OH)2D3. J Bone Miner Res 14:1478–1485
Shimamura T, Amizuka N, Li M et al (2005) Histological observations on the microenvironment of osteolytic bone metastasis by breast carcinoma cell line. Biomed Res 26:159–172
Kitazawa S, Kitazawa R (2002) RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol 198:228–236
Mancino AT, Klimberg VS, Yamamoto M et al (2001) Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J Surg Res 100:18–24
Thomas RJ, Guise TA, Yin JJ et al (1999) Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140:4451–4458
van der Pluijm G, Que I, Sijmons B et al (2005) Interference with the microenvironmental support impairs the de novo formation of bone metastases in vivo. Cancer Res 65:7682–7690
Morony S, Warmington K, Adamu S et al (2005) The RANKL inhibitor osteoprotegerin (OPG) causes greater suppression of bone resorption and hypercalcemia compared to bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology 146:3235–3243
Bendre MS, Margulies AG, Walser B et al (2005) Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 65:11001–11009
Jones DH, Nakashima T, Sanchez OH et al (2006) Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440:692–696
Gonzalez-Suarez E, Branstetter D, Armstrong A et al (2007) RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol 27:1442–1454
Roudier M, Chaisson M, Branstetter D et al (2006) RANK is expressed in human breast tumors and is functional on breast cancer cells. Breast Cancer 100(Supp 1):6124
Vanderkerken K, De Leenheer E, Shipman C et al (2003) Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res 63:287–289
Brown JE, Cook RJ, Major P et al (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97:59–69
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935
Acknowledgements
We thank Diane Duryea, Yan Cheng, Annie Luo, and Gwyneth Van for providing excellent histology and immunohistochemistry support. We thank Ji-Rong Sun for her assistance with imaging and mouse studies. We thank Tom Graves for the statistical analysis on BLI experiments. We thank Ting Chang for editorial assistance. We thank Bob Miller for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canon, J.R., Roudier, M., Bryant, R. et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 25, 119–129 (2008). https://doi.org/10.1007/s10585-007-9127-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-007-9127-1