Abstract
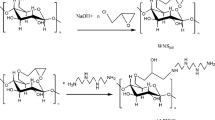
An efficient heavy metal absorbent, amino-terminated hyperbranched polymer modified walnut shell (FWNS) was prepared. The adsorption behavior for Pb(II) ions from aqueous solution was also discussed. The FWNS was prepared using an amino-terminated hyperbranched polymer (ATHBP) as modifying agent and glycidyl methacrylate(GMA) as the grafting bridge. The FWNS was characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and Scanning electron microscope (SEM). The adsorption capacity of FWNS for Pb(II) was investigated at different adsorbent dosages (0.25–2.5gL−1) and pH (from 2.0 to 6.0). The result indicated that the absorbent had good adsorption capacity over a wide pH range (2.0–6.0). The maximum Pb(II) ions adsorption capacity (Qm) obtained by a Langmuir fitting model was 1250.00 mg/g at 293 K. The adsorption kinetics fitted the pseudo-second-order equation. The adsorption of FWNS for Pb(II) ions was an endothermic reaction and spontaneous process. Furthermore, the result shows FWNS has excellent regeneration.












Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Abdel ASE, Gad YH, Dessouki AM (2006) Use of rice straw and radiation-modified maize starch/acrylonitrile in the treatment of wastewater. J Hazard Mater 129:204–212
Altun T, Pehlivan E (2012) Removal of Cr(VI) from aqueous solutions by modified walnut shells. Food Chem 132:693–700
Bhaumik M, McCrindle R, Maity A (2013) Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole-polyaniline nanofibres. Chem Eng J 228:506–515
Cao JS, Lin JX, Fang F, Zhang MT, Hu ZR (2014) A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies. Bioresour Technol 163:199–205
Chen SH, Yue QY, Gao BY, Xu X (2010) Equilibrium and kinetic adsorptions study of the adsorptive removal of Cr(VI) using modified wheat residue. J Colloid Interface Sci 349:256–264
Chen S, Yue Q, Gao B, Li Q, Xu X, Fu K (2012) Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: a fixed-bed column study. Bioresour Technol 113:114–120
Cheng S, Xing BL, Shi CL, Nie YH, Xia HY (2021) Efficient and selective removal of Pb(II) from aqueous solution by modification crofton weed: Experiment and density functional theory calculation. J Clean Prod 280(1):124407
Ding DH, Zhao YX, Yang SJ, Shi WS, Zhang ZY, Lei ZF, Yang YN (2013) Adsorption of cesium from aqueous solution using agricultural residue Walnut shell: equilibrium, kinetic and thermodynamic modeling studies. Water Res 47:2563–2571
Ding ZH, Yu R, Hu X, Chen YJ, Zhang YF (2014) Graft copolymerization of epichlorohydrin and ethylenediamine onto cellulose derived from agricultural by-products for adsorption of Pb(II) in aqueous solution. Cellulose 21:1459–1469
Eissa MM, Samy M, Ramadan AM (2015) Amino-terminated hyperbranched polymer for toughness improvement of epoxy/claynanocomposites. Polym Bull 72(12):3147–3168
Feng YF, Dionysiou DD, Wu YH, Zhou H, Yang LZ, Xue LH, He SY (2013) Adsorption of dye stuff from aqueous solutions through oxalic acid-modified swede rape straw: adsorption process and disposal methodology of depleted bioadsorbents. Bioresour Technol 138:191–197
Gong R, Zhu SX, Zhang D, Chen J, Ni SJ, Guan R (2008) Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: kinetic and thermodynamic profile. Desalination 230:220–228
Googerdchian F, Moheb A, Emadi R (2012) Lead sorption properties of nanohydro- xyapatite–alginate composite adsorbents. Chem Eng J 200–202:471–479
Jin X, Yu C, Li Y, Qi Y, Yang L, Zhao G, Hu H (2011) Preparation of novel nano-adsorbent based on organic-inorganic hybrid and their adsorption for heavy metals and organic pollutants presented in water. J Hazard Mater 186:1672–1680
Kiefer E, Sigg L, Schosseler P (1997) Chemical and spectroscopic characterization of algae surfaces. Environ Sci Technol 31:759–765
Kim SA, Kamala SK, Lee K, Park Y, Shea PJ, Lee W, Kim H, Oh B (2013) Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zero-valentiron composite. Chem Eng J 217:54–60
Lee S, Laldawngliana C, Tiwari D (2012) Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated wastewaters. Chem Eng J 195–196:103–111
Li S, Xu S, Liu S, Yang C, Lu Q (2004) Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technol 85:1201–1211
Li N, Zhang L, Chen Y, Tian Y, Wang H (2011) Adsorption behavior of Cu(II) onto titanate nanofibers prepared by alkali treatment. J Hazard Mater 189:265–272
Lin JW, Zhan YH, Zhu ZL, Xing YQ (2011) Adsorption of tannic acid from aqueous solution onto surfactant-modified zeolite. J Hazard Mater 193:102–111
Liu GT, Gao YD (2016) Synthesis, characterization of aminothiourea modified walnut Shell and its adsorption for Pb(II) ions from aqueous solution. Polymer (korea) 40(2):194–200
Liu BJ, Lv X, Meng XH, Yu GL, Wang DF (2013a) Removal of Pb(II) from aqueous solution using dithiocarbamate modified chitosan beads with Pb(II) as imprinted ions. Chem Eng J 220:412–419
Liu Y, Chen M, Hao Y (2013b) Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem Eng J 218:46–54
Liu GT, Zhang W, Luo RS (2019) Synthesis, characterization of amino-modified walnut shell and adsorption forPb(II) ions from aqueous solution. Polym Bull 76(3):1099–1114
Lo SF, Wang SY, Tsai MJ, Lin LD (2012) Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbon. Chem Eng Res Des 90:1397–1406
Mohammod M, Sen TK, Maitra S, Dutta BK (2011) Removal of Zn from aqueous solution using castor seed hull. Water Air Soil Pollut 215:609–617
Naghizadeh A, Nasseri S, Nazmara S (2011) Removal of trichloroethylene from water by adsorption on tomultiwall carbon nanotubes. Iran J Environ Health Sci Eng 8:317–325
Parab H, Sudersanan M (2010) Engineering a lignocellulosic biosorbent–coir pith for removal of cesium from aqueous solutions: equilibrium and kinetic studies. Water Res 44:854–860
Vaghetti JCP, Lima EC, Royer B, Cunha BM, Cardoso NF, Brasil JL, Dias SLP (2009) Pecan nutshell as biosorbent to remove Cu(II), Mn(II) and Pb(II) from aqueous solutions. J Hazard Mater 162:270–280
Xia CL, Jing Y, Jia YZ, Yue DY, Ma J, Yin XJ (2011) Adsorption properties of congo red from aqueous solution on modified hectorite: kinetic and thermodynamic studies. Desalination 265:81–87
Xie GR, Shang XQ, Liu RF, Hu J, Liao SF (2011) Synthesis and characterization of a novel amino modified starch and its adsorption properties for Cd(II) ions from aqueous solution. Carbohydr Polym 84:430–438
Yuvaraja G, Krishnaiah N, Subbaiah MV, Krishnaiah A (2014) Biosorption of Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids Surf b: Biointerfaces 114:75–81
Zhang F, Zhang DS, Chen YY, Lin H (2009) The antimicrobial activity of the cotton fabric grafted with an amino- terminated hyperbranched polymer. Cellulose 16(2):281–288
Acknowledgments
This work was financially supported by S & T Program of Hebei (Grant no. 21323602D), Science and Technology Research and Development Program of Qinhuangdao (Grant no. 202005A057) and the Breeding Research Subjects of Yanshan University (Grant no.16LGY020). We are thankful to Dr. Rudolph Winter, professor at Department of Chemistry&Biochemistry, University of Missouri- St Louis who made modification in English.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Zhang, L. & Luo, R. Preparation of efficient heavy metal adsorbent based on walnut shell and adsorption for Pb(II) ions from aqueous solution. Cellulose 29, 9819–9830 (2022). https://doi.org/10.1007/s10570-022-04869-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04869-z