Abstract
This work addresses a simple method to functionalize graphene oxide with sodium carboxymethyl cellulose using tetraethyl orthosilicate as a linker for rapid and significant removal of Nd(III) and Ce(III) from aqueous solutions. The prepared composite (GO–CMC) was characterized by different techniques to confirm the modification and adsorption process. The sorption performance of the GO–CMC was evaluated using Nd(III) and Ce(III) as absorbent materials. The experimental results demonstrated that the sorption process was excellently fitted by the pseudo-second-order kinetic model. The adsorption results were also analyzed by different isotherm models. According to the Langmuir isotherm model, the experimental sorption capacities at pH 3.0 was 661.21 and 436.55 mg/g for Nd(III) and Ce(III), respectively. The thermodynamic results indicated that the sorption process of the two examined metal ions was endothermic and spontaneous. The regenerated GO–CMC composite has a similar removal percentage to the original composite. These results confirmed that the prepared composite (GO–CMC) could be used as an effective adsorbent for Nd(III) and Ce(III) from certain multielement solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carboxymethyl cellulose (CMC), an anionic derivative of cellulose, contains (–CH2–COOH) groups linked to the (OH) groups of glucopyranose monomers that built the cellulose backbone. Furthermore, the CMC is an inexpensive, non-toxic, biodegradable, and renewable polymer (Noreen et al., 2020). On the other hand, GO is an oxygen-rich material containing (OH) groups located at both the GO surface and (COOH) groups localized at its edges. Several trials have been carried out to combine the GO and CMC to form an efficient structure of GO/CMC-based composites for a potential application. Huang et al. (2019) prepared composite aerogel globules based on GO and CMC, then cross-linked the globule with chitosan (CS). The as-prepared CS–GO/CMC composite aerogel recorded a maximum adsorption amount of 3190 mg/g for methylene blue, MB, and 127.4 mg/g for Cr (VI). Khawaja et al. (2021) synthesized a sponge-like porous structure (GO–CMC–Fe) composed of magnetic graphene oxide cross-linked with carboxymethyl cellulose for atrazine removal. The equilibrium adsorption process was defined by the Langmuir isotherm model with high capacities of 126, 142, 158, and 194 mg/g for GO, GO–Fe, GO–CMC, and GO–CMC–Fe, respectively. Zhao et al. (2020) fabricated 3D (CMC)/reduced graphene oxide (rGO) composite aerogel crosslinked by poly (methyl vinyl ether-co-maleic acid)/poly (ethylene glycol) for removal of MB dye. Furthermore, Eltaweil et al. (2020) prepared microbeads composed of CMC and carboxylated graphene oxide (GOCOOH) for MB dye removal. Zong et al. (2019) used a low-temperature plasma approach to graphite CMC on magnetic graphene oxide (CMC/MGOs) for U(VI) sorption. The maximum adsorption amount of U(VI) using CMC/MGOs composites, calculated from the Langmuir model, was 7.94 × 10–4 mol/g.
Moreover, Jiao et al. (2018) employed the hydrothermal treatment and ethylenediamine to graft CMC on GO for drug delivery. Allouss et al. (2019) utilized the ionotropic gelation technique to form a bead structure composed of CMC, alginate (Alg), and GO for MB dye removal. Son and Park (2018) employed a simple solution mixing-evaporation technique to prepare (CMC/GCC) nanocomposite films for improving the tensile strength and Young’s modulus of CMC film. Zhu et al. (2021) used the hydrothermal method to synthesize CMC/GO composite aerogel with a highly porous structure for adsorption of MB dye. Besides, Liu et al. (2016) employed a spray drying approach for the preparation of hydrogel microparticles (CGs) based on carboxymethyl cellulose sodium (CMCNa)/graphene oxide (GO) for removing organic and inorganic pollutants from wastewater. Juengchareonpoon et al. (2021) used citric acid to modify and crosslink (GO–CMC) nanocomposites in films to absorb antibiotics. Saladino et al. (2020) employed a solvent casting method to prepare CMC/GO biocomposite for medical applications. Additionally, Zhang et al. (2014a, b) utilized a freeze-drying approach to prepare GO/CMC with a porous structure for potential environmental applications. Varaprasad et al. (2017) utilized a free-radical polymerization approach for preparing carboxymethyl cellulose-acrylamide-graphene oxide (CMC-AM-GO) hydrogels for dye removal. Also, Chen et al. (2021) used a mixing method to prepare the magnetic CMC/GO composite for copper removal.
Since they absorb neutrons, lanthanides elements especially neodymium, have been used in nuclear applications in control rods, which are employed in nuclear reactors. In addition, the need for rare earth oxides has recently increased extremely due to technological inventions in several fields, such as therapeutic application, computer science, laser industry, metallurgy microelectronics alloys, glass that absorbs infrared wavelengths, renewable energies, military defense, and superconductors (Ponou et al. 2014; Ganjali et al. 2016). Neodymium is gaining more importance as an effective component of permanent magnets utilized in automobiles, generator motors, and hard disc drives. Different important applications for neodymium are the manufacture of lasers for use in dentistry and medicine, advanced ceramics, rubber, and superalloys. Recently, there have been numerous investigations of neodymium molecules for application in anticancer drugs (Liu et al. 2010; Feng et al. 2012).
Cerium is the most abundant element in the lanthanide elements and has many applications, such as catalysis, chemical engineering, nuclear energy, metallurgy microelectronics, luminescence, therapeutic application, and agriculture (Yantasee et al. 2009; Murthy and Choudhary 2011). Radioisotopes of cerium are the main products of nuclear fission and liquid radioactive effluents from nuclear power productions (Dubey and Rao 2011). Various techniques are available for the extraction and separation of lanthanides elements, such as liquid–liquid extraction (Xie et al. 2014), ion exchange (Moloukhia et al. 2016), co-precipitation (Dutrizac 2004), membrane separation (Dâas and Hamdaoui 2010), and adsorption. Among these techniques, the adsorption technique has been considered one of the most efficient and practical techniques due to its advantages, i.e., low cost, good renovation, simple process, high efficiency, high capacity, and no contamination (Zhao et al. 2021).
The sorption processes of some lanthanides using graphene oxide and its composites from an aqueous solution have been reviewed (Ali et al. 2019; Asadollahzadeh et al. 2021; Nazarzadeh Zare et al. 2021). Different adsorbents have been utilized for Nd(III) and Ce (III) removal, such as porous three-dimensional graphene oxide-corn zein composites (Xu et al. 2018a, b), graphene oxide nanosheets cross-linked by high-gluten flour (Xu et al. 2018a, b), HKUST-1 metal–organic framework (MOF) (Zhao et al. 2019), GO/cellulose composite films (CGC) (Hao et al. 2019), granular hydrogel composite (CTS-g-PAA/APT composite) (Zhu et al. 2015), chitosan/Polyvinyl Alcohol/3-mercaptopropyltrimethoxysilane beads (CTS/PVA/TMPTMS) (Najafi Lahiji et al. 2018), chromium-based metal–organic framework (MIL-101-PMIDA) (Lee et al. 2018), magnetic nanoparticles CuFe2O4 (Tu and Johnston 2018), graphene oxide-tris(4-aminophenyl) amine composites (GO-TAPA1:2 composite) (Zhao et al. 2021), graphene oxide nanosheets (Ashour et al. 2017) and phosphorous functionalized nanoporous carbon (Saha et al. 2017).
This work explores a modification method for modifying GO with carboxymethyl cellulose to enhance its sorption efficiency for Nd(III) and Ce(III). TEM, EDX mapping, SEM, XRD, FT-IR, and Raman, were applied to characterize the prepared GO–CMC composite. The sorption behavior of Ce(III) and Nd(III) was deeply investigated. The sorption kinetics, isotherm, and thermodynamics were also investigated to analyze the sorption mechanism. Furthermore, the obtained sorption results were compared with different adsorbent materials reported in the recent literature.
Experimental
Materials and chemicals
All chemicals and materials utilized in this study were of analytical grade and applied without purification. Ce(NO3)3·6 H2O (Sigma-Aldrich), Nd2O3 (Sigma-Aldrich), HNO3 (Sigma-Aldrich), H2SO4 (95–97%, Riedel deHaen), H2O2 (36%, Pharaohs Trading and Import, Egypt), HCl (30%, El Salam for Chemical Industries, Egypt), KMnO4 (99%, Long live), and graphite (200mesh, 99.99%, Alpha Aesar), Sodium carboxymethyl cellulose (Sigma-Aldrich), Tetraethyl orthosilicate, TEOS (99%, Across), and ethanol absolute (Sigma-Aldrich).
Preparation of Graphene oxide and GO–CMC composite
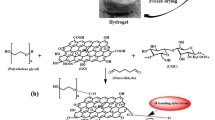
The GO was prepared according to our previous work (Abd-Elhamid et al. 2019). The GO–CMC was prepared by applying a facile one-pot blending strategy of GO, CMC, and their linker tetraethyl orthosilicate (TEOS). Briefly, 4.0 g of CMC was dissolved in 1000 mL double distilled water using a hot plate stirrer. 0.15 g of GO was added to the CMC solution and vigorously stirred to obtain a homogenous suspension of GO and CMC (Solution A). Separately, the linker was prepared by adding 10 mL of TEOS to 40 mL of ethanol absolute (solution B). Then, solution B was added drop by drop to the suspension solution A. The resultant mixture was stirred for 48 h at 60 °C. The yielded viscous product was separated by centrifugation at 6000 rpm, washed with distilled water, and stocked for further use. Scheme 1 presents a schematic clarification of GO–CMC composite preparation.
Batch adsorption studies
The stock solution of Cerium (III) (1.0 g/L) was prepared by dissolving a known amount of cerium nitrate hexahydrate Ce(NO3)3·6 H2O in deionized water. The stock solution of Nd (III) (1.0 g/L) was prepared by dissolving a known amount of the metal oxide in minimal concentrated nitric acid and evaporating it to near dryness, then formed to the mark with double-distilled water. Required concentrations of test solutions were prepared by proper dilution of the stock solutions. The concentrations of Ce(III) and Nd(III) in the different samples were determined by the Arsenazo III method at pH 3.5 in a format buffer solution at a wavelength of 650 nm (Marczenko 1976). Batch sorption experiments were performed by shaking 5.0 mL of a solution containing 50.0 mg L−1 Ce(III) and Nd(III) with 2.0 mg of the carboxymethyl cellulose modified graphene oxide in a thermostated shaker bath (G.F.L. 1083, Germany) at 25 °C. The practical experiments were performed at least three times for each experiment and the average of the results was taken. Before and after equilibration (1.0 min), known volume from the solution phase was withdrawn to determine the initial metal ion concentration before (Co) and after sorption (Ce). To avoid the formation of colloidal suspension, the adsorbent was separated from the solution by centrifugation at 5500 ± 5 rpm for 5.0 min, followed by decantation.
The capacity of carboxymethyl cellulose modified graphene oxide, qo (mg/g), was calculated using the following equation:
where Co and Ce are the initial and equilibrium concentrations (mg L−1) of metal ions in solution, respectively, V is the volume of the solution (L), and m is the weight (g) of the adsorbent.
Mathematical models
(see supplementary materials).
Instrumentation
The properties of carboxymethyl cellulose-modified graphene oxide before and after sorption were investigated using TEM, EDX, SEM, FT-IR, and Raman Spectroscopy. Transition Electron Microscope (TEM) analysis was carried out by a JEM-1010 unit (JEOL Ltd., Tokyo, Japan). The morphologies of adsorbent were identified using a JEOL SEM Model, JSM-6510A, Japan. FTIR spectra were determined by FTIR spectrometer, PerkinElmer, model 1600, USA. Elemental composition of Nd (III) and Ce (III) sorption on carboxymethyl cellulose modified graphene oxide was detected by an Oxford energy-dispersive X-ray (EDX) spectrometer (Oxford Link ISIS, Japan). Thermo-Gravimetric Analysis (TGA, Shimadzu Thermal Gravimetric Analysis (TGA)-50, Japan). A Shimadzu, UV–visible double beam spectrophotometer model UV-160A, Japan, was utilized for all spectrophotometric measurements. The interfering ions were determined by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES), Prodigy, USA.
Results and discussion
Characterization
SEM, TEM, and EDS mapping
The SEM images of GO–CMC at various magnifications (Fig. 1a-c) revealed that the GO–CMC composite owned layered structure morphology and this structure became clearer at high magnifications. To further explore the morphological and structural variation of the as-synthesized GO–CMC composite, the sample was characterized by TEM (Fig. 1 d-e). The TEM images of GO usually presented the GO in fully separated transparent. After modification of the GO with CMC, the GO–CMC is shown as a transparent sheet of GO, and a dense structure of CMC is deposited on the surface of the GO sheet, as found in Fig. 1 d-e. This unique morphology exposed a large number of the binding sites (–COO−Na+) to the toxic species, enhancing the adsorption performance and kinetics of the prepared composite. The GO carbonous material, of which the carbon sheet is made, is decorated with oxygen groups. The elemental analysis of the GO possesses C and O. With the further modification of the GO with CMC and using TEOS as a linker. The EDS analysis showed the presence of C, O, Si, and Na elements of the analyzed GO–CMC composite (Fig. S1), indicating a successful functionalization process. EDS mapping images showed uniform distribution of the O, Si, and Na over the GO sheet, which possesses a uniform layer formation of CMC over the GO sheet, see Fig. 1f-j. Therefore, the active sites are available for excellent interaction with the toxic species.
After employing the GO–CMC in the adsorption of Nd(III) and Ce(III), the GO–CMC–Ce was characterized using SEM (Fig. S2h-l). The SEM images at different magnifications described that the GO–CMC–Ce has a rigid curly layered structure, as seen in Fig. S2. Moreover, the GO–CMC-Nd was characterized using TEM (Fig. S2f-g). The TEM images showed that the soft texture of the dense structure transformed into agglomerated form, as observed in Fig.S2 a-e. This result may be attributed to the interaction between the Nd and the (–COO–Na+) of the CMC, leading to this agglomeration. It is important to observe that the GO–CMC is still a layered structure even after the adsorption process, which may improve the regeneration process of the used adsorbent.
FTIR
The FTIR spectrum was employed to assess the surface functional groups of the prepared materials. The GO spectrum possesses bands at 3433, 1635, 1398, and 1033 cm−1, referring to O–H stretching, O–H bending, COOH, and C–O stretching, respectively, as obtained in our previous work (Abd-Elhamid et al. 2018). The GO–CMC composite was prepared through a simple mixing strategy by the reaction of GO, TEOS, and CMC. Therefore, the FTIR spectrum of GO–CMC involved peaks at 3383 cm−1 (absorbed OH), 1726 cm−1 (COO−Na+, for CMC), 1636 cm−1 (bending vibration of H2O), 1415 cm−1 (deformation of carbonyl group), 1035 cm−1 (stretching vibration of C-O and Si–O)), as presented in Fig. 2a. After adsorption of Nd(III) and Ce(III) by GO–CMC to form GO–CMC-Nd and GO–CMC-Ce, respectively, some changes in the FTIR were observed. The peaks related to the absorbed water become more intense and have shifted from (3383 cm−1, GO–CMC) to (3434 cm−1, GO–CMC-Nd) and (3444 cm−1, GO–CMC–Ce). The peak at 1726 cm−1 to the disappeared carboxylated group may be attributed to the interaction between these carboxylated groups and the Nd(III) or Ce(III). The peaks at 1636 cm−1 became more intense and owned a small shift to 1629 cm−1 (in the case of Nd(III)), 1633 cm−1 (in the case of Ce(III)). Moreover, the peak at 1415 cm−1 (GO–CMC) was shifted to 1403 and 1410 cm−1 for GO–CMC–Nd and GO–CMC–Ce, respectively. Similarly, the peak at 1035 cm−1 (GO–CMC) was shifted to 1080 cm−1 after adsorption of Nd(III) or Ce(III). In addition, new peaks at 461 cm−1 were noted after the sorption of Nd(III) and Ce(III), which may be related to the M–O bond stretching (Małecka and Łącz 2008). It is noted that after adsorption of Nd(III) or Ce(III), the intensities of the peaks at 3434 and 1629 cm−1 were enhanced (consistent with the H2O motions (stretching or bending)), which may be revealed to the GO–CMC-M required water molecules to be stabilized. Finally, all variations in the FTIR spectrum suggested successful adsorption of the Nd(III) and Ce(III) over the GO–CMC adsorbent, see Fig. 2a.
Raman
Raman spectra of GO and GO–CMC are shown in Fig. 2b. The Raman spectra displayed two intense (D & G) bands. The D-band describes the defect locations related to vacancies and grain boundaries, while the G-bands correspond to the first-order scattering of the E2g of the sp2 C–C bond (Kim et al. 2013). The intensity ratio of ID/IG (sp2/sp3) decreased from 1.34 (GO) to 1.00 (GO–CMC). This result increases the σ-bond ratio, which may suggest the modification process.
XRD
The XRD pattern of GO and GO–CMC composite are shown in Fig. 3a. The XRD pattern of the GO showed the characteristic diffraction peak related to GO at 2θ = 9.4°. After interacting with CMC, this broad peak characteristic of GO disappeared, and a new diffraction broad peak was obtained at 2θ = 21.66°, which can be explained by the crosslinking effect of TEOS between GO and CMC. The obtained result was in excellent agreement with those obtained results by Ge et al. (2018).
TGA
Thermogravimetric analysis was applied to detect the decomposition weight of the analyzed material with a further increase in the temperature. The TGA analysis of GO, GO–CMC, and GO–CMC-Nd was plotted in Fig. 3b. The TGA diagram showed that GO is a thermally unstable material. More than 40% of its weight decayed at 215 oC, which corresponds to the liberation of GO water content and pyrolysis of oxygenated function groups (Zhang et al. 2014a, b), typically as obtained in our previous research (Abd-Elhamid et al. 2018). After GO with CMC modification, the GO–CMC composite showed more thermal stability than the GO, see Fig. 3b. Where the GO–CMC presents the largest decomposition percentage (14%, 35–149 °C) due to the liberation of water content. The second-largest weight reduction (12.2%, 227–428 °C) is due to pyrolysis of the polymer chain (Belmessaoud et al. 2020). As previously observed in the FTIR analysis, there was an enhancement in the water vibration bands after the adsorption process. Herein, the TGA decomposition confirmed that the GO–CMC-Nd species liberated surface and interlayer adsorbed water (20.52%, 40–221.5 °C), which was more than that liberated from the GO–CMC (18.6%, 35–223 °C), see Fig. 3b. Moreover, the further decomposition step depolymerization of CMC of the GO–CMC-Nd (12.43%, 221.5–374.5 °C) occurred at a lower temperature than GO–CMC (12.2%, 227–428 °C). This may be due to the formation of Nd2O3 as a calcination product, which acts as a catalyst to enhance the thermal decomposition process GO–CMC-Nd (Allouss et al. 2019). By further increasing the temperature to 700 °C (corresponding to the degradation of biopolymers chains and break of the C–C bonds), the GO–CMC-Nd presented a higher wt loss, % 9.9, which is ten-folds that obtained from GO–CMC (0.97), this may be referred to the catalytic action of Nd2O3 species.
EDX analysis
The preparation of GO–CMC passes through simple mixing of GO and CMC in the presence of TEOS as a linking agent. Therefore, using the EDX technique for elemental analysis of the as-synthesized composite, we observed the appearance of C, O (GO, CMC, and TEOS), Si (TEOS), and Na (CMC), as seen in Fig.S3. On the other hand, upon adsorption of GO–CMC, the metal ion (Nd(III) and Ce(III)), the analysis of the resulting complexes show the presence of these metals and a decrease in the percentage of the Na in the analysis results as shown in Fig.S3. These findings support the existence of an ion-exchange interaction between sodium GO–CMC and Nd or Ce cations.
Adsorption performance
Effect of contact time
The relation between the removal percentage (%R) of Nd(III) and Ce(III) and the contact time (1.0–30.0 min) using GO and GO–CMC as adsorbents is plotted in Fig. 4a. It was noted that the removal percentage of GO and GO–CMC reached the maximum % R in a contact time of less than 5.0 min, followed by constant percentage values. A comparison of % R for GO and GO–CMC sorbent indicated that the % R of Nd(III) and Ce(III) in the case of GO–CMC is much higher than that of GO–CMC. The reason for this fast kinetic refers to the unique structure of the composite (multi-layered structure), which allows most of the active sites exposed to the toxic species back to the SEM and TEM images of the as-prepared GO–CMC composite presented in Fig. 1. Therefore, the adsorption performance and kinetics can be improved. The parameters k2 and qe, cal (for Nd and Ce) were determined by the pseudo-second-order of the intercept and the slope of the linear (t/qt vs. t) plot, respectively, see Fig. 4b and listed in Table S1. It was noted that the calculated adsorption capacities (qe calc. mg/g) are highly close to the adsorption capacities obtained from experiments. Moreover, the R2 correlation coefficient of the two studied metal ions is ≥ 0.999 (Table S1). Furthermore, it is suggested that the pseudo-second-order model highly describes the kinetics of the adsorption process. In this context, the binding mechanism of Nd(III) and Ce(III) with GO–CMC was chemosorption (Zong et al. 2019).
Effect of a contact time on removal percent, b 2nd order kinetic model of Nd(III) ([Nd3+] = 50.0 mg L−1, Dose = 2.0 mg, V = 5.0 mL, pH = 2, T = 25 °C)) and Ce(III) ([Ce3+] = 50.0 mg L−1, Dose = 2.0 mg, V = 5.0 mL, pH = 2, T = 25 °C) from aqueous media. c Effect of the initial solution pH on the removal percent of Nd(III)(t = 1.0 min, [Nd3+] = 50.0 mg L−1, Dose = 2.0 mg, V= 5.0 mL, T = 25 °C)) and Ce(III) (t = 1.0 min, [Ce3+] = 50.0 mg L−1, Dose = 2.0 mg, V= 5.0 mL, T = 25 °C) from aqueous media
Effect of pH
The pH values of the adsorption media highly affect the adsorption efficiency because it largely induces ionization properties of the adsorbent active sites. The variation of pH values (1.0–5.0) resulted in the improvement of the removal percentage of Nd(III) (from 37.65 up to 98.77%) and Ce(III) (from 26.82 up to 87.20%), as shown in Fig. 4c. In this range, Nd(III) and Ce(III) are mainly present in the cationic form as Nd3+ and Ce3+. These metal ions can then be exchanged with sodium ions. This behavior can be explained by the fact that the binding sites of the composite are mainly composed of (–COO−Na+). Therefore, in the acidic environment, the H+-ion is found in large numbers and will compete for the metal ion species on the interacting site. This will lead to a decrease in the removal efficiency. Conversely, with a further increase in the pH value, the number of the H+ ions in the adsorption media reduced, and the ionization of the binding sites developed. This result saves suitable conditions for successful interaction among the metal ion species and the adsorbate active sites. As a result, the adsorption performance will be improved, as shown in Fig. 4c.
Effect of initial metal ion concentration
The relation between the initial metal ion concentration (50.0–500.0 mg L−1) of (Nd(III) and Ce(III)) against their removal percentage applying GO–CMC composite is given in Fig.S4a. It was observed that the removal percentage suffered from a gradual decrease, 84.75–48.72% for Nd(III) and 66.83–27.49%for Ce(III), with further increase in their initial concentrations (50–500 mg /L).
To explain the adsorption process, Langmuir, Freundlich, Dubinin-Radushkevich, Temkin, and Flory–Huggins adsorption isothermal models were employed to describe the distribution of the metal ions in the aqueous solution and the adsorbate surface (Fig. S4 b-f, Table 1). The linear correlation coefficient R2 related to the Langmuir model of both Nd(III) (R2 = 0.991) and Ce(III) (R2 = 0.975) was higher than that obtained from the other models (Table 1). This result indicated that the Langmuir isotherm model was more fit to clarify the GO–CMC homogeneous and isothermal adsorption process, which describes the favorability to adsorb Nd(III) and Ce(III) as a monolayer on the GO–CMC surface (Wu et al. 2021). Moreover, the GO–CMC can achieve experimental maximum adsorption capacities of 661.21 mg/g for Nd(III) and 436.55 mg/g for Ce(III), which were higher than that found in the literature.
Effect of adsorbent dosage
The effect of the adsorbent dosage (0.002–0.01 g) on the removal percentage of the Nd(III) and Ce(III) from aqueous media employing GO–CMC adsorbent is shown in Fig. 5a. It is worth mentioning that the removal performance linearly increases as the adsorbent dosage increase. This behavior indicates that by increasing the adsorbent dosage, the density of the available binding sites linearly increased, indicating that with this dose concentration, no agglomeration between the sorbent is observed.
Effects of a adsorbent dosage on the removal percent of Nd(III) (t = 1.0 min, [Nd3+] = 100 mg L−1, V = 5.0 mL, pH = 3, T = 25 °C)) and Ce(III) (t = 1.0 min, [Ce3+] = 100 mg L−1, V= 5.0 mL, pH = 3, T = 25 °C) from aqueous media. b Effect of temperature on the removal percent of Nd(III) ( t = 1 min, [Nd] = 100 mg L-1, Dose = 4.0 mg, V= 5.0 mL, pH = 3, T = 25 °C) and Ce(III) (t = 1 min, [Ce] = 100 mg L−1, Dose = 6.0 mg, V= 5.0 mL, pH = 3, T = 25 °C) form aqueous solution. c Effect of competing ion on the removal percent and distribution coefficient of Nd(III) and Ce(III) from a waste solution containing Ca(II), Sr(II), Cd(II) and Cs(I) ions (t = 1 min, [M] = 100 mg L−1, V/m 0.25 L/g, pH = 3, T = 25 °C). d Effect of the number of the re-use cycles of the GO–CMC on the removal percentage of Nd(III) (t = 1.0 min, [Nd] = 100 mg L−1 , Dose = 10.0 mg, V= 5.0 mL, pH = 3, T = 25 °C) and Ce(III) ([Ce] = 100 mg L−1, Dose = 10.0 mg, V= 5.0 mL, pH = 3, T = 25 °C) from aqueous media
Effect of temperature
The influence of the temperature (25–65 °C) on the interaction of Nd(III) and Ce(III) with the GO–CMC composite was depicted in Fig. 5b. The obtained data revealed that the removal percentage of Nd(III) and Ce(III) increases as the aqueous solution temperature increase. This result indicated that these metal ions adsorbed onto the GO–CMC through endothermic reactions. This behavior can be described that the increase in the temperature of the aqueous solution causing an increase in the activation energy of the metal ions, which increases the collision of the metal ions and interacting sites on the adsorbent surface, leading to an increase in the capture efficiency.
The thermodynamic studies for Nd(III) and Ce(III) adsorption on the GO–CMC (Fig.S5 and Table S2). The ΔGo values were negative at various solution temperatures, and the absolute value increased with the further temperature increase, clearing the spontaneous adsorption process of Nd(III) and Ce(III) on the GO–CMC surface. Further, the positive ΔHo value demonstrated the endothermic adsorption process. The positive value of ΔSo is associated with the improved disorder at the solid–liquid interface, indicating that the Brownian motion of metal ion molecules enhanced with the rise of temperature and improved the chaos of Nd(III) and Ce(III) adsorption by the GO–CMC (Lawal et al. 2019).
Effect of competing ions on the sorption of Nd(III) and Ce(III)
The sorption efficiency of the GO–CMC composite, as well as the distribution coefficient (Kd), was examined for the removal of Nd(III) and Ce(III) from a waste solution containing Ca(II), Sr(II), Cd(II), and Cs(I) ions was investigated, as seen in Fig. 5c. The experiment was carried out by mixing 20.0 mg of GO–CMC with a solution containing equal initial concentrations (100 mg L−1) for each ion, shaking time of 1.0 min, pH = 3.0, and V/m 0.25 L/g. These results indicated that the GO–CMC composite could be applied to remove Nd(III) and Ce(III) with high adsorption efficiency from aqueous solutions containing high ratios of interfering ions. Moreover, the Kd values for Nd(III) 54.10 and Ce(III) 2.44 L/g compared to other competing ions were less than 0.527 L/g.
Reusability of the GO–CMC adsorbent
The regeneration of GO–CMC adsorbent to reactivate the binding site was carried out using 5.0 mL of 0.50 M HCl to desorption metal ions from the binding sites, followed by washing with distilled water to remove the un-adsorbed metal ions. Finally, the GO–CMC composite was treated with 5.0 mL of 0.5 M NaOH solution and shaken for 30.0 min at 25 °C to reactivate the binding sites. Then washed with 5.0 mL distilled water to remove excess sodium hydroxide and perform the next use. The regenerated adsorbent was used in five cycles to test the removal % for Nd(III) and Ce(III), see Scheme S1. As given in Fig. 5d, it indicated approximately the same or less % removal for the metal ions under investigation.
Comparison with different adsorbent materials
It is interesting to compare the sorption capacities of modified GO–CMC for Nd(III), and Ce(III) with other adsorbent materials reported in the literature. Table 2 presents the sorption capacities of different adsorbents for Nd(III) and Ce(III). In the case of Nd(III), the highest sorption capacity reported so far is that of Gok (2014), equal to 300.0 mg/g using magnetic nano-hydroxyapatite. While for Ce(III), the maximum sorption capacity reported so far is that of Hou et al. (2008), equal to 280.0 mg/g using amino phosphonic acid chelating resin. Therefore, carboxymethyl cellulose-modified graphene oxide can be utilized as a highly efficient adsorbent for removing Nd(III) and Ce(III) from aqueous solutions.
Conclusion
Carboxymethyl cellulose modified graphene oxide (GO–CMC) was synthesized and investigated to remove Ce(III) and Nd(III) from aqueous solutions. The as-prepared composite (GO–CMC) was characterized before and after removal using TEM, SEM, FTIR, Raman, EDS mapping, TGA, XRD, and EDX analysis. The modified GO–CMC showed rapid kinetics (1.0 min) and a high adsorption capacity for Nd(III) (661.21 mg/g) and Ce(III) (436.55 mg/g). The thermodynamic results clarified that the sorption is endothermic and the process is spontaneous. The equilibrium sorption isotherms for Ce(III) and Nd(III) are fitted with the Langmuir model. The prepared composite (GO–CMC) can be used as an effective adsorbent for Nd(III) and Ce(III) from certain multielement solutions. The GO–CMC regeneration composite appears to be more or less efficient than the main composite.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Abd-Elhamid AI, Aly HF, Soliman HA, El-Shanshory AA (2018) Graphene oxide: follow the oxidation mechanism and its application in water treatment. J Mol Liq 265:226–237. https://doi.org/10.1016/j.molliq.2018.05.127
Ali I, Mbianda XY, Burakov A, Galunin E, Burakova I, Mkrtchyan E, Grachev V (2019) Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ Int 127:160–180. https://doi.org/10.1016/j.envint.2019.03.029
Allouss D, Essamlali Y, Amadine O, Chakir A, Zahouily M (2019) Response surface methodology for optimization of methylene blue adsorption onto carboxymethyl cellulose-based hydrogel beads: adsorption kinetics, isotherm, thermodynamics and reusability studies. RSC Adv 9(65):37858–37869
Asadollahzadeh M, Torkaman R, Torab-Mostaedi M (2021) Extraction and separation of rare earth elements by adsorption approaches: current status and future trends. Sep Purif Rev 50(4):417–444
Ashour RM, Abdelhamid HN, Abdel-Magied AF, Abdel-Khalek AA, Ali MM, Uheida A, Muhammed M, Zou X, Dutta J (2017) Rare earth ions adsorption onto graphene oxide nanosheets. Solvent Extr Ion Exch 35(2):91–103. https://doi.org/10.1080/07366299.2017.1287509
Awual MR, Yaita T, Shiwaku H (2013) Design a novel optical adsorbent for simultaneous ultra-trace cerium (III) detection, sorption and recovery. Chem Eng J 228:327–335. https://doi.org/10.1016/j.cej.2013.05.010
Behdani FN, Rafsanjani AT, Torab-Mostaedi M, Mohammadpour SMAK (2013) Adsorption ability of oxidized multiwalled carbon nanotubes towards aqueous Ce (III) and Sm (III). Korean J Chem Eng 30(2):448–455. https://doi.org/10.1007/s11814-012-0126-9
Belmessaoud NB, Bouslah N, Haddadine N (2020) Clay/(PEG-CMC) biocomposites as a novel delivery system for ibuprofen. J Polym Eng 40(4):350–359. https://doi.org/10.1515/polyeng-2019-0390
Chen T, Yan C, Wang Y, Tang C, Zhou S, Zhao Y, Duan P (2015) Synthesis of activated carbon-based amino phosphonic acid chelating resin and its adsorption properties for Ce (III) removal. Environ Technol 36(17):2168–2176. https://doi.org/10.1080/09593330.2015.1023365
Chen J, Luo W, Guo A, Luo T, Lin C, Li H, Jing L (2017) Preparation of a novel carboxylate-rich palygorskite as an adsorbent for Ce3+ from aqueous solution. J Colloid Interface Sci 512:657–664. https://doi.org/10.1016/j.jcis.2017.09.107
Chen Y, Cui J, Liang Y, Chen X, Li Y (2021) Synthesis of magnetic carboxymethyl cellulose/graphene oxide nanocomposites for adsorption of copper from aqueous solution. Int J Energy Res 45(3):3988–3998. https://doi.org/10.1002/er.6054
Dâas A, Hamdaoui O (2010) Extraction of anionic dye from aqueous solutions by emulsion liquid membrane. J Hazard Mater 178(1–3):973–981. https://doi.org/10.1016/j.jhazmat.2010.02.033
Demir S, Brune NK, Van Humbeck JF, Mason JA, Plakhova TV, Wang S, Long JR (2016) Extraction of lanthanide and actinide ions from aqueous mixtures using a carboxylic acid-functionalized porous aromatic framework. ACS Cent Sci 2(4):253–265. https://doi.org/10.1021/acscentsci.6b00066
Dubey SS, Rao BS (2011) Removal of cerium ions from aqueous solution by hydrous ferric oxide – a radiotracer study. J Hazard Mater 186(2–3):1028–1032. https://doi.org/10.1016/j.jhazmat.2010.11.085
Dutrizac JE (2004) The behaviour of the rare earths during the precipitation of sodium, potassium and lead jarosites. Hydrometallurgy 73(1–2):11–30. https://doi.org/10.1016/j.hydromet.2003.07.009
Eltaweil AS, Elgarhy GS, El-Subruiti GM, Omer AM (2020) Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int J Biol Macromol 154:307–318. https://doi.org/10.1016/j.ijbiomac.2020.03.122
Feng C, Gan Q, Liu X, He H (2012) Synthesis and antitumor activities of rare earth substituted phosphotungstates containing 5-fluorouracil. J Rare Earths 30(5):467–472. https://doi.org/10.1016/S1002-0721(12)60074-X
Fu Q, Yang L, Wang Q (2007) On-line preconcentration with a novel alkyl phosphinic acid extraction resin coupled with inductively coupled plasma mass spectrometry for determination of trace rare earth elements in seawater. Talanta 72(4):1248–1254. https://doi.org/10.1016/j.talanta.2007.01.015
Galhoum AA, Mahfouz MG, Abdel-Rehem ST, Gomaa NA, Atia AA, Vincent T, Guibal E (2015) Diethylenetriamine-functionalized chitosan magnetic nano-based particles for the sorption of rare earth metal ions [Nd (III), Dy (III) and Yb (III)]. Cellulose 22(4):2589–2605. https://doi.org/10.1007/s10570-015-0677-0
Ganjali MR, Gupta VK, Faridbod F, Norouzi P (2016) Applications of the lanthanide series in human life. Lanthanides series determination by various analytical methods. Elsevier, London, pp 37–58
Ge X, Shan Y, Wu L, Mu X, Peng H, Jiang Y (2018) High-strength and morphology-controlled aerogel based on carboxymethyl cellulose and graphene oxide. Carbohydr Polym 197:277–283. https://doi.org/10.1016/j.carbpol.2018.06.014
Gok C (2014) Neodymium and samarium recovery by magnetic nano-hydroxyapatite. J Radioanal Nucl Chem 301(3):641–651. https://doi.org/10.1007/s10967-014-3193-z
Hao Y, Cui Y, Peng J, Zhao N, Li S, Zhai M (2019) Preparation of graphene oxide/cellulose composites in ionic liquid for Ce (III) removal. Carbohydr Polym 208:269–275. https://doi.org/10.1016/j.carbpol.2018.12.068
Hou LX, Jiang F, Wang S (2008) Synthesis and application of an amino phosphonic acid chelating resin for adsorption of Cerium (III). J Anal Chem 63(4):337–341. https://doi.org/10.1134/S1061934808040059
Huang T, Shao YW, Zhang Q, Deng YF, Liang ZX, Guo FZ, Wang Y (2019) Chitosan-cross-linked graphene oxide/carboxymethyl cellulose aerogel globules with high structure stability in liquid and extremely high adsorption ability. ACS Sustain Chem Eng 7(9):8775–8788. https://doi.org/10.1021/acssuschemeng.9b00691
Jiao Z, Zhang B, Li C, Kuang W, Zhang J, Xiong Y, Tan S, Cai X, Huang L (2018) Carboxymethyl cellulose-grafted graphene oxide for efficient antitumor drug delivery. Nanotechnol Rev 7(4):291–301. https://doi.org/10.1515/ntrev-2018-0029
Juengchareonpoon K, Wanichpongpan P, Boonamnuayvitaya V (2021) Graphene oxide and carboxymethylcellulose film modified by citric acid for antibiotic removal. J Environ Chem Eng 9(1):104637. https://doi.org/10.1016/j.jece.2020.104637
Karadaş C, Kara D, Fisher A (2011) Determination of rare earth elements in seawater by inductively coupled plasma mass spectrometry with off-line column preconcentration using 2, 6-diacetylpyridine functionalized Amberlite XAD-4. Anal Chim Acta 689(2):184–189. https://doi.org/10.1016/j.aca.2011.01.049
Keçili R, Dolak İ, Ziyadanoğulları B, Ersöz A, Say R (2018) Ion imprinted cryogel-based supermacroporous traps for selective separation of cerium (III) in real samples. J Rare Earths 36(8):857–862. https://doi.org/10.1016/j.jre.2018.02.008
Khawaja H, Zahir E, Asghar MA, Rafique K, Asghar MA (2021) Synthesis and application of covalently grafted magnetic graphene oxide carboxymethyl cellulose nanocomposite for the removal of atrazine from an aqueous phase. J Macromol Sci B 60(12):1025–1044. https://doi.org/10.1080/00222348.2021.1949515
Kim SG, Park OK, Lee JH, Ku BC (2013) Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett Carbon 14(4):247–250. https://doi.org/10.5714/CL.2013.14.4.247
Lawal IA, Lawal MM, Azeez MA, Ndungu P (2019) Theoretical and experimental adsorption studies of phenol and crystal violet dye on carbon nanotube functionalized with deep eutectic solvent. J Mol Liq 288:110895. https://doi.org/10.1016/j.molliq.2019.110895
Lee YR, Yu K, Ravi S, Ahn WS (2018) Selective adsorption of rare earth elements over functionalized Cr-MIL-101. ACS Appl Mater Interfaces 10(28):23918–23927. https://doi.org/10.1021/acsami.8b07130
Li C, Pan J, Gao J, Yan Y (2009) A novel Ce (III)-imprinted polymer supported by attapulgite: synthesis, characterisation and adsorption behaviours towards Ce (III) in aqueous solution. Int J Mater Struct Integr 3(4):294–308. https://doi.org/10.1504/IJMSI.2009.029337
Lin C, Luo W, Chen J, Zhou Q (2017) Rice husk grafted PMAA by ATRP in aqueous phase and its adsorption for Ce3+. J Chem Phys 690:68–73. https://doi.org/10.1016/j.cplett.2017.10.029
Liu X, Wang S, Feng C (2010) Synthesis and anticancer properties of tungstosilicic polyoxometalate containing 5-fluorouracil and neodymium. J Rare Earths 28(6):965–968. https://doi.org/10.1016/S1002-0721(09)60227-1
Liu J, Chu H, Wei H, Zhu H, Wang G, Zhu J, He J (2016) Facile fabrication of carboxymethyl cellulose sodium/graphene oxide hydrogel microparticles for water purification. RSC Adv 6(55):50061–50069. https://doi.org/10.1039/C6RA06438H
Małecka B, Łącz A (2008) Thermal decomposition of cadmium formate in inert and oxidative atmosphere. Thermochim Acta 479(1–2):12–16. https://doi.org/10.1016/j.tca.2008.09.003
Marczenko Z (1976) Spectrophotometric determination of elements. Ellis Harwood Ltd, Poland
Moloukhia H, Hegazy WS, Abdel-Galil EA, Mahrous SS (2016) Removal of Eu3+, Ce3+, Sr2+, and Cs+ ions from radioactive waste solutions by modified activated carbon prepared from coconut shells. J Chem Ecol 32(4):324–345. https://doi.org/10.1080/02757540.2016.1139089
Murthy ZVP, Choudhary A (2011) Separation of cerium from feed solution by nanofiltration. Desalination 279(1–3):428–432. https://doi.org/10.1016/j.desal.2011.06.014
Najafi Lahiji M, Keshtkar AR, Moosavian MA (2018) Adsorption of cerium and lanthanum from aqueous solutions by chitosan/polyvinyl alcohol/3mercaptopropyltrimethoxysilane beads in batch and fixed-bed systems. Part Sci Technol 36(3):340–350. https://doi.org/10.1080/02726351.2016.1248262
Naser AA, El-deen GS, Bhran AA, Metwally SS, El-Kamash AM (2015) Elaboration of impregnated composite for sorption of europium and neodymium ions from aqueous solutions. J Ind Eng Chem 32:264–272. https://doi.org/10.1016/j.jiec.2015.08.024
Nazarzadeh Zare E, Mudhoo A, Ali Khan M, Otero M, Bundhoo ZMA, Patel M, Srivastava A, Navarathna C, Mlsna T, Mohan D, Pittman CU Jr, Makvandi P, Sillanpää M (2021) Smart adsorbents for aquatic environmental remediation. Small 17(34):2007840
Noreen A, Tabasum S, Ghaffar S, Somi T, Sultan N, Aslam N, Naseer R, Ali I, Anwar F (2020) Protein-based bionanocomposites. Bionanocompos Green Synth Appl Micro Nano Technol. https://doi.org/10.1016/B978-0-12-816751-9.00012-X
Ponou J, Wang LP, Dodbiba G, Okaya K, Fujita T, Mitsuhashi K, Atarashi T, Satoh G, Noda M (2014) Recovery of rare earth elements from aqueous solution obtained from Vietnamese clay minerals using dried and carbonized parachlorella. J Environ Chem Eng 2(2):1070–1081
Roosen J, Binnemans K (2014) Adsorption and chromatographic separation of rare earths with EDTA-and DTPA-functionalized chitosan biopolymers. J Mater Chem A 2(5):1530–1540. https://doi.org/10.1039/C3TA14622G
Saha D, Akkoyunlu SD, Thorpe R, Hensley DK, Chen J (2017) Adsorptive recovery of neodymium and dysprosium in phosphorous functionalized nanoporous carbon. J Environ Chem Eng 5(5):4684–4692. https://doi.org/10.1016/j.jece.2017.09.009
Saladino ML, Markowska M, Carmone C, Cancemi P, Alduina R, Presentato A, Wawrzyńska M (2020) Graphene oxide carboxymethylcellulose nanocomposite for dressing materials. Materials 13(8):1980. https://doi.org/10.3390/ma13081980
Son YR, Park SJ (2018) Green preparation and characterization of graphene oxide/carbon nanotubes-loaded carboxymethyl cellulose nanocomposites. Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-35984-2
Su S, Chen B, He M, Hu B, Xiao Z (2014) Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@ SiO2@ polyaniline–graphene oxide composite. Talanta 119:458–466. https://doi.org/10.1016/j.talanta.2013.11.027
Tu YJ, Johnston CT (2018) Rapid recovery of rare earth elements in industrial wastewater by CuFe2O4 synthesized from Cu sludge. J Rare Earths 36(5):513–520. https://doi.org/10.1016/j.jre.2017.11.009
Varaprasad K, Jayaramudu T, Sadiku ER (2017) Removal of dye by carboxymethyl cellulose, acrylamide and graphene oxide via a free radical polymerization process. Carbohydr Polym 164:186–194. https://doi.org/10.1016/j.carbpol.2017.01.094
Wu Z, Deng W, Tang S, Ruiz-Hitzky E, Luo J, Wang X (2021) Pod-inspired MXene/porous carbon microspheres with ultrahigh adsorption capacity towards crystal violet. J Chem Eng 426:130776. https://doi.org/10.1016/j.cej.2021.130776
Xie F, Zhang TA, Dreisinger D, Doyle F (2014) A critical review on solvent extraction of rare earths from aqueous solutions. Miner Eng. 56:10–28. https://doi.org/10.1016/j.mineng.2013.10.021
Xu X, Jiang XY, Jiao FP, Chen XQ, Yu JG (2018a) Tunable assembly of porous three-dimensional graphene oxide-corn zein composites with strong mechanical properties for adsorption of rare earth elements. J Taiwan Inst Chem Eng 85:106–114
Xu X, Zou J, Teng J, Liu Q, Jiang XY, Jiao FP, Chen XQ (2018b) Novel high-gluten flour physically cross-linked graphene oxide composites: hydrothermal fabrication and adsorption properties for rare earth ions. Ecotoxicol Environ Saf 166:1–10. https://doi.org/10.1016/j.ecoenv.2018.09.062
Yan P, He M, Chen B, Hu B (2017) Fast preconcentration of trace rare earth elements from environmental samples by di (2-ethylhexyl) phosphoric acid grafted magnetic nanoparticles followed by inductively coupled plasma mass spectrometry detection. Spectrochim Acta B: At Spectrosc 136:73–80. https://doi.org/10.1016/j.sab.2017.08.011
Yanfei XIAO, Huang L, Zhiqi LONG, Zongyu FENG, Liangshi WANG (2016) Adsorption ability of rare earth elements on clay minerals and its practical performance. J Rare Earths 34(5):543–548. https://doi.org/10.1016/S1002-0721(16)60060-1
Yantasee W, Fryxell GE, Addleman RS, Wiacek RJ, Pattamakomsan K, Sukwarotwat V, Raymond KN (2009) Selective removal of lanthanides from natural waters, acidic streams and dialysate. J Hazard Mater 168(2–3):1233–1238. https://doi.org/10.1016/j.jhazmat.2009.03.004
Zhang L, Wu D, Zhu B, Yang Y, Wang L (2011) Adsorption and selective separation of neodymium with magnetic alginate microcapsules containing the extractant 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. J Chem Eng Data 56(5):2280–2289. https://doi.org/10.1021/je101270j
Zhang H, Zhai D, He Y (2014a) Graphene oxide/polyacrylamide/carboxymethyl cellulose sodium nanocomposite hydrogel with enhanced mechanical strength: preparation, characterization and the swelling behavior. RSC Adv 4(84):44600–44609. https://doi.org/10.1039/C4RA07576E
Zhang Y, Liu Y, Wang X, Sun Z, Ma J, Wu T, Xing F, Gao J (2014b) Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohydr Polym 101:392–400. https://doi.org/10.1016/j.carbpol.2013.09.066
Zhao L, Azhar MR, Li X, Duan X, Sun H, Wang S, Fang X (2019) Adsorption of cerium (III) by HKUST-1 metal-organic framework from aqueous solution. J Colloid Interface Sci 542:421–428. https://doi.org/10.1016/j.jcis.2019.01.117
Zhao M, Zhang S, Fang G, Huang C, Wu T (2020) Directionally-grown carboxymethyl cellulose/reduced graphene oxide aerogel with excellent structure stability and adsorption capacity. Polymers 12(10):2219. https://doi.org/10.3390/polym12102219
Zhao X, Jiang X, Peng D, Teng J, Yu J (2021) Behavior and mechanism of graphene oxide-tris (4-aminophenyl) amine composites in adsorption of rare earth elements. J Rare Earths 39(1):90–97. https://doi.org/10.1016/j.jre.2020.02.006
Zhou S, Li X, Shi Y, Alshameri A, Yan C (2015) Preparation, characterization, and Ce (III) adsorption performance of poly (allylamine)/silica composite. Desalin Water Treat 56(5):1321–1334. https://doi.org/10.1080/19443994.2014.944221
Zhu Y, Zheng Y, Wang A (2015) Preparation of granular hydrogel composite by the redox couple for efficient and fast adsorption of La (III) and Ce (III). J Environ Chem Eng 3(2):1416–1425. https://doi.org/10.1016/j.jece.2014.11.028
Zhu W, Jiang X, Jiang K, Liu F, You F, Yao C (2021) Fabrication of reusable carboxymethyl cellulose/graphene oxide composite aerogel with large surface area for adsorption of methylene blue. Nanomaterials 11(6):1609. https://doi.org/10.3390/nano11061609
Zong P, Cao D, Cheng Y, Wang S, Zhang J, Guo Z, He C (2019) Carboxymethyl cellulose supported magnetic graphene oxide composites by plasma induced technique and their highly efficient removal of uranium ions. Cellulose 26(6):4039–4060. https://doi.org/10.1007/s10570-019-02358-4
Acknowledgments
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
EMAE: Conceptualization, Data curation, Investigation, Methodology, Writing—original draft, writing – review, and editing. AIAE: Conceptualization, Data curation, Methodology, Software, Supervision, Writing—original draft, Writing – review, and editing. HFA: Conceptualization, Data curation, Investigation, Supervision, Writing – review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors approved the paper submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd-Elhamid, A.I., Abu Elgoud, E.M. & Aly, H.F. Graphene oxide modified with carboxymethyl cellulose for high adsorption capacities towards Nd(III) and Ce(III) from aqueous solutions. Cellulose 29, 9831–9846 (2022). https://doi.org/10.1007/s10570-022-04862-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04862-6