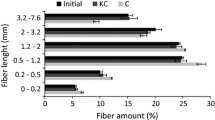
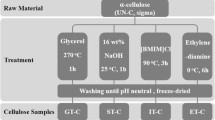
Abstract
The enzymatic hydrolysis of cotton raw cellulose (RC) samples, sieved RC samples through meshes <100 (CS1), 100–200 (C12), 200–400 (C24), mercerized RC samples (M-C), freeze-dried RC (RC-FD) samples, microcrystalline cellulose Avicel, bacterial cellulose (BC), raw sisal pulp and mercerized sisal pulp (S-M) was performed at cellulose-to-cellulase mass ratios of 1,000:1, 699:1, 400:1, 100:1 and 10:1. The index of crystallinity and water sorption values were quantified for all samples. The morphological features were analyzed by means of scanning electron microscopy (SEM). For cellulose-to-cellulase mass ratio of 100:1 and 10:1, the maximum hydrolysis extents of cellulose samples after 24 h reaction could not be correlated with their physical characteristics. However, hydrolyses of samples with large water sorption values were faster than those with lower water sorption values. The hydrolysis efficiency decreased when the cellulose-to-cellulase mass ratio was greater than 400:1; under this condition a remarkable dependence of the hydrolysis yield on the type of cellulosic sample was observed. The water sorption ability could be directly correlated with the hydrolysis extent, except for RC-FD and BC samples, which presented the lowest values. In the former, freeze-drying has led to pore collapse, with concomitant reduction of the amount of adsorbed water. For the latter sample, the densely packed structure made the water sorption slower than in all other samples. Despite of this fact, the presence of nanofibrils on the surface of BC (as detected by SEM) improved the enzyme adsorption, indicating that analysis by complementary techniques should be performed in order to predict the enzymatic hydrolysis efficiency.







Similar content being viewed by others
References
Andrić P, Meyer AS, Jensen PA, Dam-Johansen K (2010) Effect and modeling of glucose inhibition and in situ glucose removal during enzymatic hydrolysis of pretreated wheat straw. Appl Biochem Biotechnol 160:280–297
Arantes V, Saddler JN (2011) Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol Biofuels 4:3–16
Bansal P, Hall M, Realff MJ, Lee JH, Bommarius AS (2009) Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol Adv 27:833–848
Bansal P, Hall M, Realff MJ, Lee JH, Bommarius AS (2010) Multivariate statistical analysis of x-ray data from cellulose: a new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour Technol 101:4461–4471
Bansal P, Vowell BJ, Hall M, Realff MJ, Lee JH, Bommarius AS (2012) Elucidation of cellulose accessibility, hydrolysability and reactivity as the major limitations in the enzymatic hydrolysis of cellulose. Bioresour Technol 107:243–250
Bezerra RMF, Dias AA (2004) Discrimination among eight modified Michaelis-Menten kinetics models of cellulose hydrolysis with a large range of substrate/enzyme ratios: inhibition by cellobiose. Appl Biochem Biotechnol 112:173–184
Brown RM Jr (1996) The biosynthesis of cellulose. Macromol Sci Pure Appl Chem A33:1345–1373
Cao Y, Tan H (2005) Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzyme Microb Technol 36:314–317
Chandra RP, Ewanick SM, Chung PA, Au-Yeung K, Del Rio L, Mabee W, Saddler JN (2009) Comparison of methods to assess the enzyme accessibility and hydrolysis of pretreated lignocellulosic substrates. Biotechnol Lett 31:1217–1222
Ciolacu D, Gorgieva S, Tampu D, Kokol V (2011) Enzymatic hydrolysis of different allomorphic forms of microcrystalline cellulose. Cellulose 18:1527–1541
Coughlan MP (1985) The properties of fungal and bacterial cellulases with comment on their production and application. In: Russell GE (ed) Biotechnology and genetic engineering reviews, vol 3. Interscience, Newcastle-upon-Tyne, pp 37–109
Driemeier C, Mendes FM, Oliveira MM (2012) Dynamic vapor sorption and thermoporometry to probe water in celluloses. Cellulose 19:1051–1063
El Seoud O, Ludmila CF, Ruiz N, D’Almeida MLO, Frollini E (2008) Cellulose swelling by protic solvents: which properties of the biopolymer and the solvent matter? Cellulose 18:371–392
Fan LT, Lee YH, Beardmore DR (1981) Mechanism of enzymatic hydrolysis of cellulose: effects of major structural features of cellulose on enzyme hydrolysis. Biotechnol Bioeng 23:419–424
Fidale LC, Ruiz N, Heinze T, El Seoud OA (2008) Cellulose swelling by aprotic and protic solvents: what are the similarities and differences? Macromol Chem Phys 209:1240–1254
Galet L, Patry S, Dodds J (2010) Determination of the wettability of powders by the Washburn capillary rise method with bed preparation by a centrifugal packing technique. J. Colloid Interf Sci 346:470–475
Griggs AJ, Stickel JJ, Lischeske JJ (2012) A mechanistic model for enzymatic saccharification of cellulose using continuous distribution kinetics I: depolymerization by EGI and CBH. Biotechnol Bioeng 109:665–675
Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS (2010) Cellulose crystallinity—a key predictor of the enzymatic hydrolysis rate. FEBS J 277:1571–1582
Hatakeyama T, Nakamura K, Hatakeyama H (2000) Vaporization of bound water associated with cellulose fibers. Thermochim Acta 352–353:233–239
Jeoh T, Ishizawa CI, Davis MF, Himmel ME, Adney WS, Johnson DK (2007) Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol Bioeng 98:112–122
Ko CH, Chen FJ, Lee JJ, Tzou DLM (2011) Effects of fiber physical and chemical characteristics on the interaction between endoglucanase and eucalypt fibers. Cellulose 18:1043–1054
Kobayashi K, Kimura S, Kim UJ, Tokuyasu K, Wada M (2012) Enzymatic hydrolysis of cellulose hydrates. Cellulose 19:967–974
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Lacerda TM, de Paula MP, Zambon M, Frollini E (2012) Saccharification of Brazilian sisal pulp: evaluating the impact of mercerization on non-hydrolyzed pulp and hydrolysis products. Cellulose 19:351–362
Levine SE, Fox JM, Blanch HW, Clark DS (2010) A mechanistic model of the enzymatic hydrolysis of cellulose. Biotechnol Bioeng 107:37–51
Levine SE, Fox JM, Clark DS, Blanch HW (2011) A mechanistic model for rational design of optimal cellulase mixtures. Biotechnol Bioeng 108:2561–2570
Luo XL, Zhu JY, Gleisner R, Zhan HY (2011) Effects of wet-pressing-induced fiber hornification on enzymatic saccharification of lignocelluloses. Cellulose 18:1055–1062
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Mansfield SD, Mooney C, Saddler JN (1999) Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol Prog 15:804–816
Mazeau K (2011) On the external morphology of native cellulose microfibrils. Carbohydr Polym 84:524–532
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mizutani C, Inagaki H, Bertoniere NR (1999) Water absorbency of never-dried cotton fibers. Cellulose 6:167–176
Ogeda TL, Silva IB, Fidale LC, El Seoud OA, Petri DFS (2012) Effect of cellulose physical characteristics, especially the water sorption value, on the efficiency of its hydrolysis catalyzed by free or immobilized cellulase. J Biotechnol 157:246–252
Persin Z, Stana-Kleinschek K, Kreze T (2002) Hydrophilic/hydrophobic characteristics of different cellulose fibres monitored by tensiometry. Croat Chem Acta 75:271–280
Pu Y, Ziemer C, Ragauskas AJ (2006) CP/MAS 13C NMR analysis of cellulase treated bleached softwood kraft pulp. Carbohydrate Res 341:591–597
Puri VP (1984) Effect of crystallinity and degree of polymerization of cellulose on enzymatic saccharification. Biotechnol Bioeng 26:1219–1222
Ramos LA, Morgado DL, El Seoud OA, da Silva VC, Frollini E (2011) Acetylation of cellulose in LiCl-N, N-dimethylacetamide: first report on the correlation between the reaction efficiency and the aggregation number of dissolved cellulose. Cellulose 18:385–392
Roberts KM, Lavenson DM, Tozzi EJ, McCarthy MJ, Jeoh T (2011) The effects of water interactions in cellulose suspensions on mass transfer and saccharification efficiency at high solids loadings. Cellulose 18:759–773
Sattler W, Esterbauer H, Glatter O, Steiner W (2004) The effect of enzyme concentration on the rate of the hydrolysis of cellulose. Biotechnol Bioeng 33:121–1234
Sewalt VJH, Beauchemin KA, Rode LM (1997a) Lignin impact on fiber degradation. IV. Enzymatic saccharification and in vitro digestibility of Alfalfa and grasses following selective solvent delignification. Bioresour Technol 61:199–206
Sewalt VJH, Glasser WG, Beauchemin KA (1997b) Lignin impact on fiber degradation. 3. reversal of inhibition of enzymatic hydrolysis by chemical modification of lignin and by additives. J Agric Food Chem 45:1823–1828
Strømme M, Mihranyan A, Ek R, Niklasson GA (2003) Fractal dimension of cellulose powders analyzed by multilayer BET adsorption of water and nitrogen. J Phys Chem B 107:14378–14382
Tengborg C, Galbe M, Zacchi G (2001) Reduced inhibition of enzymatic hydrolysis of steam-pretreated softwood. Enzyme Microbial Technol 28:835–844
Topalovic T, Nierstrasz VA, Bautista VL, Jocic D, Navarro A, Warmoeskerken MMCG (2007) XPS and contact angle study of cotton surface oxidation by catalytic bleaching. Colloids Surf A.: physicochem. Eng Aspects 296:76–85
Vanderghem C, Jacquet N, Danthine S, Blecker C, Paquot M (2012) Effect of physicochemical characteristics of cellulosic substrates on enzymatic hydrolysis by means of a multi-stage process for cellobiose production. Appl Biochem Biotechnol 166:1423–1432
Wang W, Kang L, Wei H, Arora R, Lee YY (2011) Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Appl Biochem Biotechnol 164:1139–1149
Weast RC (1983). Handbook of physical chemistry, 64th ed. CRC Press Inc
Wyman CE, Decker SR, Himmel ME, Brady JW, Skopec CE (2005) Hydrolysis of cellulose and hemicellulose. In: Dumitriu S (ed) Polysaccharides: Structural diversity and functional versatility. Dekker, New York, pp 995–1034
Xiao Z, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and β-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 157:113–116
Yu X, Atalla RH (1998) A staining technique for evaluating the pore structure variations of microcrystalline cellulose powders. Powder Technol 98:135–138
Yu Z, Jameel H, Chang H, Philips R, Park S (2012) Evaluation of the factors affecting avicel reactivity using multi-stage enzymatic hydrolysis. Biotechnol Bioeng 109:1131–1139
Yui T, Ogawa K (2005) X-Ray diffraction study of polysaccharides. In: Dumitriu S (ed) Polysaccharides: Structural diversity and functional versatility. Dekker, New York, pp 99–122
Zhang YHP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng 88:797–824
Zhang YHP, Lynd LR (2005) Determination of the number-average degree of polymerization of cellodextrins and cellulose with application to enzymatic hydrolysis. Biomacromolecules 6:1510–1515
Acknowledgments
E. Frollini, O. A. El Seoud and D. F. S. Petri thank the CNPq research foundation for research productivity fellowships; T. Stauner and I. B. Silva thank CAPES-Rede Nanobiotec and CNPq for fellowships, we thank Rede Nanobiotec CAPES and FAPESP (Grant # 2010/51219-4) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stauner, T., Silva, I.B., El Seoud, O.A. et al. Cellulose loading and water sorption value as important parameters for the enzymatic hydrolysis of cellulose. Cellulose 20, 1109–1119 (2013). https://doi.org/10.1007/s10570-013-9904-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-9904-8