Abstract
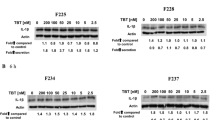
Natural killer (NK) cells destroy (lyse) tumor cells, virally infected cells, and antibody-coated cells. Previous studies indicated that exposure to the environmental contaminant tributyltin (TBT) decreases the lytic function of NK cells and activates mitogen-activated protein kinases (MAPK), including p44/42 (Aluoch and Whalen Toxicology 209:263–277, 2005). If activation of p44/42 is required for TBT-induced decreases of lytic function, then activation of p44/42 to similar extents by pharmacological agents such as phorbol 12-myristate 13-acetate (PMA) should mimic to some extent changes induced in NK cells with TBT exposures. NK cells were exposed to PMA concentrations between 0.25 and 10 nM for 10 min, 1 h, and 6 h before determining the lytic function (51Cr release assay) and phosphorylation state of MAPKs (Western blot). A 1-h exposure of NK cells to 5 nM PMA resulted in a loss of lytic function of 47%. Western blot analysis showed that a 1-h exposure to 5 nM PMA caused a sixfold increase in phospho-p44/42 levels. Previous studies showed a fivefold increase in phospho-p44/42 in response to a 1-h exposure to 300 nM TBT. Exposure to 300 nM TBT caused about a 40% decrease in lytic function. This study supports the hypothesis that p44/42 activation (as seen with TBT exposures) can cause a loss of NK-cell lytic function.





Similar content being viewed by others
References
Abraha A, Whalen MM. The role of p44/42 activation in tributyltin-induced inhibition of human natural killer cells: effects of MEK inhibitors. J Appl Toxicol. 2009;29:165–73.
Aluoch AO, Whalen MM. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells. Toxicology. 2005;209:263–77.
Aluoch AO, Odman-Ghazi SO, Whalen MM. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–37.
Aluoch AO, Odman-Ghazi SO, Whalen MM. Pattern of MAP kinases p44/42 and JNK activation by non-lethal doses of tributyltin in human natural killer cells. Arch Toxicol. 2007;81:271–7.
Biron CA, Byron KS, Sullivan JL. Severe herpes virus in an adolescent without natural killer cells. New Engl J Med. 1989;320:1731–5.
Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effect of tributyltin (TBT) on the ATP levels in human natural killer cells: relationship to TBT- induced decreases in NK function. J Appl Toxicol. 2007;27:86–94.
Fleisher G, Koven N, Kamiya H, Henle W. A non-X-linked syndrome with susceptibility to severe Epstein–Barr virus infections. J Pediatr. 1982;100:727–30.
Hanna N. Expression of metastatic potential of tumor cells in young nude mice is correlated with low levels of natural-killer cell mediated cytotoxicity. Int J Cancer. 1980;26:675–90.
Hanson RD, Grisolano JL, Lay TJ. Consensus AP-1 and CRE motifs upstream from the human cytotoxic serine protease B (CSP-B/CGL-1) gene synergizes to activate transcription. Blood. 1993;82:2749–57.
Kannan K, Tanabe S, Tatsukawa R. Occurrence of butyltin residues in certain foodstuffs. Bull Environ Contam Toxicol. 1995a;55:510–6.
Kannan K, Tanabe S, Tatsukawa R, Williams RJ. Butyltin residues in fish from Australia, Papua New Guinea and the Solomon Islands. Int J Environ Anal Chem. 1995b;61:263–73.
Kannan K, Tanabe S, Iwata H, Tatsukawa R. Butyltins in muscle and liver of fish collected from certain Asian and Oceanian countries. Environ Pollut. 1995c;90:279–90.
Kannan K, Senthilkumar K, Giesy JP. Occurrence of butyltin compounds in human blood. Environ Sci Technol. 1999;33:1776–9.
Kiessling R, Haller O. Natural killer cells in the mouse, an alternative surveillance mechanism? Contemp Top Immunobiol. 1978;8:171–201.
Kimbrough RD. Toxicity and health effects of selected organotins compounds: a review. Environ Health Perspect. 1976;14:51–6.
Lane R, Ghazi SO, Whalen MM. Increases in cytosolic calcium ion levels in human natural killer cells in response to butyltin exposure. Arch Environ Contam Toxicol. 2009;57(4):816–25.
Laughlin RB, Linden O. Fate and effects of organotin compounds. Ambio. 1985;14:88–94.
Loganathan BG, Kannan K, Owen DA, Sajwan KS. Butyltin compounds in freshwater ecosystems. In: Lipnick RL, Hermens J, Jones K, Muir D, editors. Persistent, bioaccumulative, and toxic chemicals. I Fate and Exposure, Am. Chem. Soc. London: Oxford University Press; 2000.
Lotzova E. Definition and function of natural killer cells. Natural Immun. 1993;12:177–93.
Middlebrook JL, Leatherman DL. Binding of T-2 toxin to eukaryotic cell ribosomes. Biochem Pharmacol. 1989;38:3103–10.
O’Shea J, Ortaldo JR. The biology of natural killer cells: insight into the molecular basis of function. In: Lewis CE, McGee JOD, editors. The natural killer cell. Oxford: IRL; 1992. p. 1–40.
Roper WL. Toxicological profile for tin. USA: U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry; 1992.
Shifrin VI, Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem. 1999;274:13985–92.
Tanabe S, Prudente M, Mizuno T, Hasegawa J, Iwata H, Miyazaki N. Butyltin contamination in marine mammals from north Pacific and Asian coastal waters. Environ Sci Technol. 1998;32:193–8.
Thomas LD, Shah H, Green SA, Bankhurst AD, Whalen MM. Tributyltin exposure causes decreased granzyme B and perforin levels in human natural killer cells. Toxicology. 2004;200:221–33.
Thomas LD, Shah H, Bankhurst AD, Whalen MM. Effects of interleukins 2 and 12 on the levels of granzyme B and perforin and their mRNAs in tributyltin-exposed human natural killer cells. Arch Toxicol. 2005;79:711–20.
Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376.
Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–9.
Whalen MM. Inhibition of human natural killer cell function in vitro by glucose concentrations seen in poorly controlled diabetes. Cell Physiol Biochem. 1997;7:53–60.
Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environ Res. 1999;81:108–16.
Whalen MM, Williams TB, Green SA, Loganathan BG. Interleukins 2 and 12 produce recovery of cytotoxic function in tributyltin-exposed human natural killer cells. Environ Res. 2002;88:189–209.
Whalen MM, Loganathan BG, Yamashita N, Saito T. Immunomodulation of human natural killer cell cytotoxic function by triazine and carbamate pesticides. Chemico-Biological Int. 2003;145:311–9.
Yamada S, Fuji Y, Mikami E, Kawamura N, Hayakawa J. Small-scale survey of organotin compounds in household commodities. J AOAC Int. 1993;76:436–41.
Acknowledgment
This research was supported by Grant 2S06GM-08092-34 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dudimah, F.D., Griffey, D., Wang, X. et al. Activation of p44/42 MAPK plays a role in the TBT-induced loss of human natural killer (NK) cell function. Cell Biol Toxicol 26, 435–444 (2010). https://doi.org/10.1007/s10565-010-9154-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-010-9154-6