Abstract
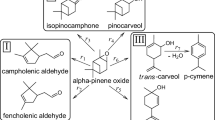
Isomerization of α-pinene epoxide was carried out over a Colombian natural zeolite as potential geocatalyst with heulandite, chabazite and clipnotilolite as main crystallographic phases; the heulandite was identified as the active phase. Over synthetic zeolites, isomerization of α-pinene epoxide depended on Si/Al ratio, unit cell and the kind of structure. The best solvent was toluene and not isomerization activity was observed in presence of solvents with carboxyl groups. Complete α-pinene epoxide conversion and 45% selectivity to campholenic aldehyde were obtained at 70 °C, with fencholenic aldehyde, carveol, and p-cymene as main by-products. Decrease of activity of natural zeolite was associated with loss of acid sites. A reaction mechanism based on experimental and computational data was proposed including adsorption of α-pinene epoxide on Fe or Al sites of the natural zeolite; a reaction rate constant of 4.02 × 10–4 mol g−1 min−1 was estimated from a pseudo homogeneous kinetic model.
Graphic Abstract



















Similar content being viewed by others
References
Magdziarz A, Gajek M, Nowak-Woźny D et al (2018) Renew Energy 128:446–459
Deng L, Ye J, Jin X et al (2017) Energy Procedia 142:401–406
Park J, Meng J, Lim K, Rojas O, Park S (2013) J Anal Appl Pyrolysis 100:199–206
Breitmaier E (1997) Common fragrance and flavor materials
Kim M, Sowndhararajan K, Park S et al (2018) Eur J Integr Med 17:33–39
Qu L, Yu H, Yu F et al (2018) Appl Surf Sci 453:271–279
Becerra J, Villa A (2018) Chem Eng Technol 41:124–133
Tao P, Lu X, Zhang H et al (2019) Mol Catal 463:8–15
Meyer-Waßewitz J, Elyorgun JD, Conradi C et al (2018) Chem Eng Res Des 134:463–475
Patil M, Yadav M, Jasra R (2007) J Mol Catal A 277:72–80
Tang B, Lu X, Zhou D et al (2012) Catal Commun 21:68–71
Liebens A, Mahaim C, Holderich W (1997) Heterog Catal Fine Chem Iv 108:587–594
Bruno S, Gomes A, Abrantes M et al (2015) J Organomet Chem 799–800:179–183
Frater G, Bajgrowicz J (1998) Optical isomers of derivatives of campholenic aldehyde. Eur Pat Appl
Medina F, Tichit D, Pe J (2007) App Catal B 70:577–584
Schulze K, Uhlig H (1989) Chem Mon 120:547–559
Guo J, Zhang R, Ouyang J et al (2018) Chem Cat Chem 10:5496–5504
Kaminska J, Schwegler M, Hoefnagel A et al (1992) Recl des Trav Chim des Pays-Bas 111:432–437
Stekrova M, Kumar N, Aho A et al (2014) Appl Catal A 470:162–176
Shcherban N, Barakov R, Mäki-Arvela P et al (2018) Appl Catal A 560:236–247
Sidorenko A, Kravtsova A, AhobI A et al (2018) Mol Catal 448:18–29
Timofeeva M, Panchenko V, Hasan Z et al (2014) Catal A Gen 469:427–433
Ravasio N, Zaccheria F, Gervasini A et al (2008) Catal Commun 9:1125–1127
Villa-Holguín A, Sánchez-Velandia J (2018) Rev Colomb Química 47:13–23
Pitínová-Štekrová M, Eliášová P, Weissenberger T et al (2018) Catal Sci Technol 8:4690–4701
Wang S, Peng Y (2010) Chem Eng J 156:11–24
Wibowo E (2017) Procedia Eng 170:8–13
Ates A, Akgül G (2016) Powder Technol 287:285–291
Wang S, Ariyanto E (2007) J Colloid Interface Sci 314:25–31
Gelves J, Dorkis L, Márquez M et al (2018) Catal Today 320:112–122
Merissa S, Fitriani P, Iskandar F, Abdullah M, Khairurrijal (2013) AIP Conf. Proc 1554:131–134
Wijayati N, Utomo A (2016) Int J Chem Eng Appl 7:138–141
Sharma P, Singh G et al (2009) J Colloid Interface Sci 332:298–308
Bates SA, Verma AA, Paoulucci C et al (2014) J Catal 312:87–97
Sánchez-Velandia J, Villa-Holguín A, Gelves J et al (2019) Microporous Mesoporous Mater 287:114–123
Steven J, Khasanov A, Miller J et al (2005) Mössbauer mineral handbook. Mössbauer Effect Data Center, Asheville
Goldanskii V, Herber RH (1968) Chemical application of the Móssbauer spectroscopy. Academic Press, New York
Giannini C, Ladisa M, Altamura D et al (2016) Crystals 6(8):87
Gelves JF, Gallego GS, Marquez MA (2016) Microporous Mesoporous Mater 235:9–19
Alberti A (1973) Mineral Petrol 19(3):173–184
Passaglia E, Sheppard RA (2011) Rev Mineral Geochem 45(1):69–116
Viczian I (2013) Földvári, Mária: Occasional Papers of the Geological Institute of Hungary, vol 213, Budapest, 2011
Conte M, Lopez-Sanchez JA et al (2012) Catal Sci Technol 2(1):105–112
Sui GJ, Sun QL, Wu D et al (2016) RSC Adv 6(68):63493–63496
Dalton Research Group. Dielectric constants of common organic solvents. Dep. Chem. Univ. Washington. 1. https://depts.washington.edu/eooptic/linkfiles/dielectric_chart%5B1%5D.pdf, Accessed 18 Dec 2018
Anslyn E, Dougherty D (2006) Modern physic organic chemistry. Remington Farmacia 91.
Wishart D, Arndt D, Pon A et al (2015) T3DB: the toxic exposome database. Nucleic Acids Res 43:D928–D934
Value of pKaTable, University of Massachusetts. Available on:owl.oit.umass.edu. Accessed 14 Jan 2019
Riddick J, Bunger W, Sakano T (1985) Techniques of chemistry, 4th ed, vol II. Organic solvents, p. 309
Kilner C, Halcrow A (2006) Crystal structure communications. Acta Crystallogr C C62:M437–M439
Chan A, Harvey B, Hoggard P (2013) Photochem Photobiol Sci 12(9):1680–1687
Tsai W (2017) Toxics 5(4):23–35
Coelho J, de Meireles A, da Silva RK et al (2012) Appl Catal A 443–444:125–132
Stekrova M, Kubu M, Shamzhy M et al (2018) Catal Sci Technol 9:2488–2501
Sánchez-Velandia J, Villa A (2019) Appl Catal A 580:17–27
Sánchez-Velandia J, Agudelo-Cifuentes A, Villa A (2019) React Kinet Mech Catal 128(2):1005–1028
Timofeeva M, Panchenko V, Hasan Z et al (2014) Appl Catal A 469:427–433
Davis E, Davis R (2003) Fundamentals of chemical reaction engineering. McGraw-Hili Chemical Engineering Series, vol. 43
Casado J, Lopez-Quintela MA, Lorenzo-Barral FM (1986) J Chem Educ 63:450
Kinetic model hydroformylation. Departament of Chemistry, Texas A&M University. Available on: https://www.chem.tamu.edu/rgroup/marcetta/chem462/lectures/Cho-Sanchez-Hydroformylation.pdf. Accessed 01 May 2019
Vicevic M, Boodhoo K, Scott K (2007) Chem Eng J 133:31–41
Saminen E, Maki-Arvela P, Virtanen P et al (2014) Ind Eng Chem Res 53:20107–20115
Maki-Arvela P, Scherban N, Lozachemeur C et al (2019) Catal Lett 149:203–214
Heulandite phase A: General Information. Springer materials. https://materials.springer.com/isp/crystallographic/docs/sd_1602809. Accessed 12 Dec 2018
Acknowledgements
The authors acknowledge to COLCIENCIAS and Universidad de Antioquia (UdeA) for the financial support through the contract 059-2016. J.E. S.-V. acknowledges to COLCIENCIAS for his fellowship (call 785) and the Instructor program from UdeA. J.F-G acknowledges to COLCIENCIAS for his fellowship (call 528 -2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Velandia, J.E., Gelves, J.F., Márquez, M.A. et al. Catalytic Isomerization of α-Pinene Epoxide Over a Natural Zeolite. Catal Lett 150, 3132–3148 (2020). https://doi.org/10.1007/s10562-020-03225-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03225-9