Abstract
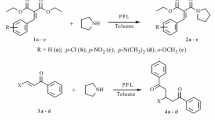
Lipase from porcine pancreas (PPL) is first reported to catalyze direct 1,6-conjugated addition for synthesis of triarylmethanes using p-quinonemethides (p-QMs) and 2-naphthols. The catalytic activity of PPL was evaluated through investigating the solvent, the ratio of substrates, the enzyme loading and the temperature of the enzyme-catalyzed reactions. The present method proves to be environmentally friendly and efficient in terms of high yield, green catalyst and simple synthesis method.
GraphicAbstract







Similar content being viewed by others
References
Duxbury DF (1993) The photochemistry and photophysics of triphenylmethane dyes in solid and Liquid-Media. Chem Rev 93(1):381–433
Shiri M, Zolfigol MA, Kruger HG, Tanbakouchian Z (2010) Bis- and trisindolylmethanes (BIMs and TIMs). Chem Rev 110(4):2250–2293
Wang TL, Hong TT, Huang Y, Su HM, Wu F, Chen Y et al (2015) Fluorescein derivatives as bifunctional molecules for the simultaneous inhibiting and labeling of FTO protein. J Am Chem Soc 137(43):13736–13739
Al-Qawasmeh RA, Lee Y, Cao MY, Gu XP, Vassilakos A, Wright JA et al (2004) Triaryl methane derivatives as antiproliferative agents. Bioorg Med Chem Lett 14(2):347–350
Mibu N, Yokomizo K, Uyeda M, Sumoto K (2005) Synthesis and antiviral activities of some 4,4’- and 2,2’-dihydroxytriphenylmethanes. Chem Pharm Bull 53(9):1171–1174
Mereyala HB, Sambaru K (2005) Synthesis of triphenylmethane derivative: bisacodyl. Indian J Chem B 44(3):615–617
Parai MK, Panda G, Chaturvedi V, Manju YK, Sinha S (2008) Thiophene containing triarylmethanes as antitubercular agents. Bioorg Med Chem Lett 18(1):289–292
Singh P, Manna SK, Jana AK, Saha T, Mishra P, Bera S et al (2015) Thiophene containing trisubstitutedmethanes [TRSMs] as identified lead against Mycobacterium tuberculosis. Eur J Med Chem 95:357–368
Manabe K, Mori Y, Wakabayashi T, Nagayama S, Kobayashi S (2000) Organic synthesis inside particles in water: lewis acid-surfactant-combined catalysts for organic reactions in water using colloidal dispersions as reaction media. J Am Chem Soc 122(30):7202–7207
Wilsdorf M, Leichnitz D, Reissig HU (2013) Trifluoromethanesulfonic acid catalyzed friedel-crafts alkylations of 1,2,4-trimethoxybenzene with aldehydes or benzylic alcohols. Org Lett 15(10):2494–2497
Bacci JP, Kearney AM, Van Vranken DL (2005) Efficient two-step synthesis of 9-aryl-6-hydroxy-3H-xanthen-3-one fluorophores. J Org Chem 70(22):9051–9053
Nambo M, Crudden CM (2015) Recent advances in the synthesis of triarylmethanes by transition metal catalysis. ACS Catal 5(8):4734–4742
Kapoor M, Gupta MN (2012) Lipase promiscuity and its biochemical applications. Process Biochem 47(4):555–569
Aouf C, Durand E, Lecomte J, Figueroa-Espinoza MC, Dubreucq E, Fulcrand H et al (2014) The use of lipases as biocatalysts for the epoxidation of fatty acids and phenolic compounds. Green Chem 16(4):1740–1754
Kirchner G, Scollar MP, Klibanov AM (1985) resolution of racemic mixtures via lipase catalysis in organic-solvents. J Am Chem Soc 107(24):7072–7076
Bornscheuer UT, Kazlauskas RJ (2004) Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways. Angew Chem Int Ed 43(45):6032–6040
Jaeger KE, Reetz MT (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol 16(9):396–403
Svedendahl M, Hult K, Berglund P (2005) Fast carbon-carbon bond formation by a promiscuous lipase. J Am Chem Soc 127(51):17988–17989
Torre O, Alfonso I, Gotor V (2004) Lipase catalysed Michael addition of secondary amines to acrylonitrile. ChemCommun 15:1724–1725
Wu WB, Xu JM, Wu Q, Lv DS, Lin XF (2006) Promiscuous acylases-catalyzed Markovnikov addition of N-heterocycles to vinyl esters in organic media. Adv Synth Catal 348(4–5):487–492
Carboni-Oerlemans C, de Maria PD, Tuin B, Bargeman G, van der Meer A, van Gemert R (2006) Hydrolase-catalysed synthesis of peroxycarboxylic acids: biocatalytic promiscuity for practical applications. J Biotechnol 126(2):140–151
Svedendahl M, Carlqvist P, Branneby C, Allner O, Frise A, Hult K et al (2008) Direct epoxidation in candida antarctica lipase b studied by experiment and theory. ChemBioChem 9(15):2443–2451
Branneby C, Carlqvist P, Magnusson A, Hult K, Brinck T, Berglund P (2003) Carbon-carbon bonds by hydrolytic enzymes. J Am Chem Soc 125(4):874–875
Lai YF, Zheng H, Chai SJ, Zhang PF, Chen XZ (2010) Lipase-catalysed tandem Knoevenagel condensation and esterification with alcohol cosolvents. Green Chem 12(11):1917–1918
He YH, Li HH, Chen YL, Xue Y, Yuan Y, Guan Z (2012) Chymopapain-catalyzed direct asymmetric aldol reaction. Adv Synth Catal 354(4):712–719
Wang JL, Li X, Xie HY, Liu BK, Lin XF (2010) Hydrolase-catalyzed fast Henry reaction of nitroalkanes and aldehydes in organic media. J Biotechnol 145(3):240–243
Li C, Feng XW, Wang N, Zhou YJ, Yu XQ (2008) Biocatalytic promiscuity: the first lipase-catalysed asymmetric aldol reaction. Green Chem 10(6):616–618
Xie ZB, Wang N, Jiang GF, Yu XQ (2013) Biocatalytic asymmetric aldol reaction in buffer solution. Tetrahedron Lett 54(8):945–948
Zhang Y, Wang N, Xie ZB, Zhou LH, Yu XQ (2014) Ionic liquid as a recyclable and efficient medium for lipase-catalyzed asymmetric cross aldol reaction. J MolCatal B-Enzyme 110:100–110
Li K, He T, Li C, Feng XW, Wang N, Yu XQ (2009) Lipase-catalysed direct Mannich reaction in water: utilization of biocatalytic promiscuity for C-C bond formation in a “one-pot” synthesis. Green Chem 11(6):777–779
He T, Li K, Wu MY, Feng XW, Wang N, Wang HY et al (2010) Utilization of biocatalytic promiscuity for direct Mannich reaction. J Mol Catal B-Enzyme 67(3–4):189–194
Zhou LH, Wang N, Chen GN, Yang Q, Yang SY, Zhang W et al (2014) Lipase-catalyzed highly diastereoselective direct vinylogous Michael addition reaction of alpha, alpha-dicyanoolefins to nitroalkenes. J Mol Catal B-Enzyme 109:170–177
Zhou LH, Wang N, Zhang W, Xie ZB, Yu XQ (2013) Catalytical promiscuity of alpha-amylase: synthesis of 3-substituted 2H-chromene derivatives via biocatalytic domino oxa-Michael/aldol condensations. J Mol Catal B-Enzyme 91:37–43
Zhang W, Wang N, Yang ZJ, Li YR, Yu Y, Pu XM et al (2017) Lipase-initiated tandem biginelli reactions via in situ-formed acetaldehydes in one pot: discovery of single-ring deep blue luminogens. Adv Synth Catal 359(19):3397–3406
Acknowledgements
This work was financially supported by the National Key R&D Program of China (Grant No. 2018YFA0901600). We also thank the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, ZJ., Wang, N., He, WX. et al. Lipase-Catalyzed Highly Efficient 1,6-Conjugated Addition for Synthesis of Triarylmethanes. Catal Lett 150, 1268–1276 (2020). https://doi.org/10.1007/s10562-019-03043-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-03043-8