Abstract
A series of N-cycloalkylprolinamides have been designed and synthesized from achiral cycloalkylamines in a facile manner. They promoted high stereoselectivity in the cross-aldol reaction. N-cyclopropylprolinamide performed best with a smallest carbocyclic ring, and the anti-aldol products could be obtained with up to 99:1 anti/syn and 99% ee. Carbocyclic ring was found to play a significant role in the formation of the aldol products. This simple catalyst can be efficiently used in large-scale reactions with the enantioselectivity being maintained at the same level, which offers great possibility for applications in industry.
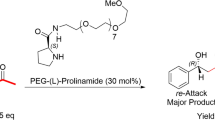
Graphical Abstract
All of the catalysts 1a–e synthesized from achiral cycloalkylamines in a facile manner and their catalytic properties were studied in depth for the first time. All of the catalysts 1a–e exhibited great catalytic activity in the asymmetric aldol reaction in m-xylene using acetic acid as cocatalyst at −20 °C with only 5 mol% catalyst loading in 4 equivalents ketone, and the anti-aldol products could be obtained with up to 99:1 anti/syn and 99% ee. Compared with proline and prolinamide 1f, it is easily found that the carbocyclic ring of N-cycloalkyl-l-prolinamide could be anticipated in some way to enhance the enantioselectivity in m-xylene at low temperature. The results observed for 1a surpass those reported so far for simple prolinamide derivatives and are comparable to those for organocatalysts of much more structural complexity. Catalyst 1a can be efficiently used in large-scale reactions with the enantioselectivity being maintained at the same level, which offers great possibility for applications in industry.


Similar content being viewed by others
References
Berkessel A, Gröger H (eds) (2005) Books on organocatalysis: asymmetric organocatalysis: from biomimetic concepts to applications in asymmetric synthesis. Wiley-VCH, Weinheim
Dalko PI (ed) (2007) Enantioselective organocatalysis: reactions and experimental procedures. Wiley-VCH, Weinheim
Tian SK, Chen YG, Hang JF, Tang L, McDaid P, Deng L (2004) Acc Chem Res 37:621
List B (2007) Chem Rev 107:5413
Enders D, Grondal C, Hüttl RM (2007) Angew Chem Int Ed 46:1570
Dondoni A, Massi A (2008) Angew Chem Int Ed 47:4638
Notz W, Tanaka F, Barbas CF III (2004) Acc Chem Res 37:580
Limbach M (2005) Chem Biodiv 2:825
Schetter B, Mahrwald R (2006) Angew Chem Int Ed 45:7506
MacMillan DWC (2008) Nature 455:304
Dalko PL, Moisan L (2004) Angew Chem Int Ed 43:5138
Li CJ (2005) Chem Rev 105:3095
Palomo M, Oiarbide JM (2004) Garca Chem Soc Rev 33:65
Alcaide B, Almendros P (2003) Angew Chem Int Ed 42:858
Mukherjee S, Yang JW, Hoffmann S, List B (2007) Chem Rev 107:5471
List B, Lerner RA, Barbas CF III (2000) J Am Chem Soc 122:2395
Bahmanyar S, Houk KN, Martin HJ, List B (2003) J Am Chem Soc 125:2475
List B, Hoang L, Martin HJ (2004) Proc Natl Acad Sci USA 101:5839
Chen JR, Liu XP, Zhu XY, Li L, Zhang JM, Qiao YF, Xiao WJ (2007) Tetrahedron 63:10437
Huang WP, Chen JR, Li XY, Cao YJ, Xiao WJ (2007) Can J Chem 85:208
Guizzetti S, Benaglia M, Raimondi L, Celentano G (2007) Org Lett 9:1247
Guillena G, Nájera C, Viózquez SF (2008) Synlett 19:3031
Guillena G, Hita MDC, Nájera C, Vióquez SF (2008) J Org Chem 73:5933
Mase N, Tanaka F, Barbas CF III (2003) Org Lett 5:4369
Thayumanavan R, Tanaka F, Barbas CF III (2004) Org Lett 6:3541
Utsumi N, Imai M, Tanaka F, Ramasastry SSV, Barbas CF III (2007) Org Lett 9:3445
Zhong GF, Fan JH, Barbas CF III (2004) Tetrahedron Lett 45:5681
Aratake S, Itoh T, Okano T, Nagae N, Sumiya T, Shoji M, Hayashi Y (2007) Chem Eur J 13:10246
Aratake S, Itoh T, Okano T, Usui T, Shoji M, Hayashi Y (2007) Chem Commun 24:2524
Hayashi Y, Aratake S, Itoh T, Okano T, Sumiya T, Shoji M (2007) Chem Commun 9:957
Hayashi Y, Aratake S, Okano T, Takahashi J, Sumiya T, Shoji M (2006) Angew Chem Int Ed 45:5527
Tang Z, Jiang F, Yu LT, Cui X, Gong LZ, Qiao A, Jiang YZ, Wu YD (2003) J Am Chem Soc 125:5262
Tang Z, Jiang F, Yu LT, Cui X, Gong LZ, Mi AQ, Jiang YZ, Wu YD (2004) Proc Natl Acad Sci USA 101:5775
Tang Z, Yang ZH, Cun LF, Gong LZ, Mi AQ, Jiang YZ (2004) Org Lett 6:2285
Tang Z, Yang ZH, Chen XH, Cun LF, Mi AQ, Jiang YZ, Gong LZ (2005) J Am Chem Soc 127:9285
He L, Tang Z, Cun LF, Mi AQ, Jiang YZ, Gong LZ (2005) Tetrahedron 62:346
Chen JR, Lu HH, Li XY, Cheng L, Wan J, Xiao WJ (2005) Org Lett 7:4543
Chen JR, Li XY, Xing XN, Xiao WJ (2006) J Org Chem 71:8198
Gury Z, Isra GHY (2004) J Org Chem 69:4966
Tsutomu M (2000) Chem Pharm Bull 48:1310
Zheng CS, Andrew C (2002) P J Comb Chem 4:38
Acknowledgments
Authors are grateful to Southwest University of China for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, JW., Fu, XK., Hu, XY. et al. Simple and Facile l-Prolinamides Derived from Achiral Cycloalkylamines as Organocatalysts for the Highly Efficient Large-Scale Asymmetric Direct Aldol Reactions. Catal Lett 141, 1156–1163 (2011). https://doi.org/10.1007/s10562-011-0585-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0585-3