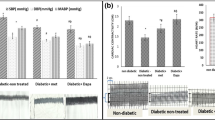
Abstract
Background
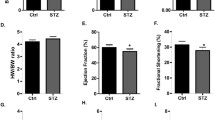
Dapagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, is approved for the treatment of type 2 diabetes, heart failure, and chronic kidney disease. DAPA-HF and DELIVER trial results demonstrate that the cardiovascular protective effect of dapagliflozin extends to non-diabetic patients. Hence, the mechanism-of-action may extend beyond glucose-lowering and is not completely elucidated. We have previously shown that dapagliflozin reduces cardiac hypertrophy, inflammation, fibrosis, and apoptosis and increases ejection fraction in BTBR mice with type 2 diabetes.
Methods
We conducted a follow-up RNA-sequencing study on the heart tissue of these animals and performed differential expression and Ingenuity Pathway analysis. Selected markers were confirmed by RT-PCR and Western blot.
Results
SGLT2 had negligible expression in heart tissue. Dapagliflozin improved cardiac metabolism by decreasing glycolysis and pyruvate utilization enzymes, induced antioxidant enzymes, and decreased expression of hypoxia markers. Expression of inflammation, apoptosis, and hypertrophy pathways was decreased. These observations corresponded to the effects of dapagliflozin in the clinical trials.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
RNA-sequencing read counts and differential expression results (Supplementary Table 1), IPA pathway analysis results without p-value cut-off (Supplementary Table 2), and z-score transformed expression values for Fig. 2 (Supplementary Table 3) are enclosed with this article. Raw FASTQ files could not be recovered.
References
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. https://doi.org/10.1056/NEJMoa1812389.
Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors' expert forum. Diabetes Care. 2018;41(1):14–31. https://doi.org/10.2337/dci17-0057.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Inzucchi SE, Claggett BL, Vaduganathan M, et al. Efficacy and safety of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction by baseline glycaemic status (DELIVER): a subgroup analysis from an international, multicentre, double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2022;10(12):869–81. https://doi.org/10.1016/S2213-8587(22)00308-4.
Joubert M, Jagu B, Montaigne D, et al. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes. 2017;66(4):1030–40. https://doi.org/10.2337/db16-0733.
Arow M, Waldman M, Yadin D, et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol. 2020;19(1):7. https://doi.org/10.1186/s12933-019-0980-4.
Saleh S, Hanna G, El-Nabi SH, et al. Dapagliflozin, a sodium glucose cotransporter 2 inhibitors, protects cardiovascular function in type-2 diabetic murine model. J Genet. 2020;99:1–8. https://doi.org/10.1007/s12041-020-01196-9.
Olgar Y, Turan B. A sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin comparison with insulin shows important effects on Zn(2+)-transporters in cardiomyocytes from insulin-resistant metabolic syndrome rats through inhibition of oxidative stress. Can J Physiol Pharmacol. 2019;97(6):528–35. https://doi.org/10.1139/cjpp-2018-0466.
Shi L, Zhu D, Wang S, Jiang A, Li F. Dapagliflozin attenuates cardiac remodeling in mice model of cardiac pressure overload. Am J Hypertens. 2019;32(5):452–9. https://doi.org/10.1093/ajh/hpz016.
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 Inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc Drugs Ther. 2017;31(2):119–32. https://doi.org/10.1007/s10557-017-6725-2.
Chen H, Tran D, Yang HC, et al. Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: an AMPK-mTOR interplay. Cardiovasc Drugs Ther. 2020;34(4):443–61. https://doi.org/10.1007/s10557-020-06978-y.
Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. https://doi.org/10.1093/bioinformatics/bts635.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. https://doi.org/10.1186/s13059-014-0550-8.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2016.
Mak TW, Hauck L, Grothe D, Billia F. p53 regulates the cardiac transcriptome. Proc Natl Acad Sci USA. 2017;114(9):2331–6. https://doi.org/10.1073/pnas.1621436114.
Croteau D, Luptak I, Chambers JM, et al. Effects of sodium-glucose linked transporter 2 inhibition with ertugliflozin on mitochondrial function, energetics, and metabolic gene expression in the presence and absence of diabetes mellitus in mice. J Am Heart Assoc. 2021;10(13):e019995. https://doi.org/10.1161/JAHA.120.019995.
Zhang S, Liu H, Amarsingh GV, et al. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc Diabetol. 2014;13:100. https://doi.org/10.1186/1475-2840-13-100.
Uthman L, Kuschma M, Romer G, et al. Novel anti-inflammatory effects of canagliflozin involving hexokinase II in lipopolysaccharide-stimulated human coronary artery endothelial cells. Cardiovasc Drugs Ther. 2021;35(6):1083–94. https://doi.org/10.1007/s10557-020-07083-w.
Nair V, Schaub J, Alakwaa F, et al. WCN23-0761 SGLT2 inhibitor treatment may enhance kidney oxygenation and attenuate HIF1a expression in young persons with type 2 diabetes. Kindney Int Rep. 2023;8(3):S197–S8. https://doi.org/10.1016/j.ekir.2023.02.439.
Nagatomo Y, Meguro T, Ito H, et al. Significance of AT1 receptor independent activation of mineralocorticoid receptor in murine diabetic cardiomyopathy. PLoS One. 2014;9(3):e93145. https://doi.org/10.1371/journal.pone.0093145.
Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab (Lond). 2009;6:33. https://doi.org/10.1186/1743-7075-6-33.
Mushtaq I, Bashir Z, Sarwar M, et al. N-acetyl cysteine, selenium, and ascorbic acid rescue diabetic cardiac hypertrophy via mitochondrial-associated redox regulators. Molecules. 2021;26(23) https://doi.org/10.3390/molecules26237285.
Kain V, Kumar S, Sitasawad SL. Azelnidipine prevents cardiac dysfunction in streptozotocin-diabetic rats by reducing intracellular calcium accumulation, oxidative stress and apoptosis. Cardiovasc Diabetol. 2011;10:97. https://doi.org/10.1186/1475-2840-10-97.
Ding W, Chang WG, Guo XC, et al. Exenatide protects against cardiac dysfunction by attenuating oxidative stress in the diabetic mouse heart. Front Endocrinol (Lausanne). 2019;10:202. https://doi.org/10.3389/fendo.2019.00202.
Cotrin JC, de Souza GSM, Petito-da-Silva TI, et al. Empagliflozin alleviates left ventricle hypertrophy in high-fat-fed mice by modulating renin angiotensin pathway. J Renin Angiotensin Aldosterone Syst. 2022;2022:8861911. https://doi.org/10.1155/2022/8861911.
Oshima H, Miki T, Kuno A, et al. Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther. 2019;368(3):524–34. https://doi.org/10.1124/jpet.118.253666.
Nabrdalik-Lesniak D, Nabrdalik K, Sedlaczek K, et al. Influence of SGLT2 inhibitor treatment on urine antioxidant status in type 2 diabetic patients: a pilot study. Oxid Med Cell Longev. 2021;2021:5593589. https://doi.org/10.1155/2021/5593589.
Xue W, Liu Y, Zhao J, et al. Activation of HIF-1 by metallothionein contributes to cardiac protection in the diabetic heart. Am J Physiol Heart Circ Physiol. 2012;302(12):H2528–35. https://doi.org/10.1152/ajpheart.00850.2011.
Kim S, Jo CH, Kim GH. Effects of empagliflozin on nondiabetic salt-sensitive hypertension in uninephrectomized rats. Hypertens Res. 2019;42(12):1905–15. https://doi.org/10.1038/s41440-019-0326-3.
Bessho R, Takiyama Y, Takiyama T, et al. Hypoxia-inducible factor-1alpha is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep. 2019;9(1):14754. https://doi.org/10.1038/s41598-019-51343-1.
Ghanim H, Abuaysheh S, Hejna J, et al. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020;105(4) https://doi.org/10.1210/clinem/dgaa057.
Peake BF, Nicholson CK, Lambert JP, et al. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol. 2013;304(9):H1215–24. https://doi.org/10.1152/ajpheart.00796.2012.
Li C, Zhang J, Xue M, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15. https://doi.org/10.1186/s12933-019-0816-2.
Xia Y, Gong L, Liu H, et al. Inhibition of prolyl hydroxylase 3 ameliorates cardiac dysfunction in diabetic cardiomyopathy. Mol Cell Endocrinol. 2015;403:21–9. https://doi.org/10.1016/j.mce.2015.01.014.
Han B, Baliga R, Huang H, Giannone PJ, Bauer JA. Decreased cardiac expression of vascular endothelial growth factor and redox imbalance in murine diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2009;297(2):H829–35. https://doi.org/10.1152/ajpheart.00222.2009.
Gui C, Zeng ZY, Chen Q, et al. Neuregulin-1 promotes myocardial angiogenesis in the rat model of diabetic cardiomyopathy. Cell Physiol Biochem. 2018;46(6):2325–34. https://doi.org/10.1159/000489622.
Zhang WY, Wang J, Li AZ. A study of the effects of SGLT-2 inhibitors on diabetic cardiomyopathy through miR-30d/KLF9/VEGFA pathway. Eur Rev Med Pharmacol Sci. 2020;24(11):6346–59. https://doi.org/10.26355/eurrev_202006_21533.
Wang M, Lv Q, Zhao L, et al. Metoprolol and bisoprolol ameliorate hypertrophy of neonatal rat cardiomyocytes induced by high glucose via the PKC/NF-kappaB/c-fos signaling pathway. Exp Ther Med. 2020;19(2):871–82. https://doi.org/10.3892/etm.2019.8312.
Nguyen T, Wen S, Gong M, et al. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting SGLT-2 in mice. Diabetes Metab Syndr Obes. 2020;13:2781–99. https://doi.org/10.2147/DMSO.S258593.
Raut SK, Singh GB, Rastogi B, et al. miR-30c and miR-181a synergistically modulate p53-p21 pathway in diabetes induced cardiac hypertrophy. Mol Cell Biochem. 2016;417(1-2):191–203. https://doi.org/10.1007/s11010-016-2729-7.
Cheng Y, Li J, Wang C, et al. Inhibition of long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 attenuates high glucose-induced cardiomyocyte apoptosis via regulation of miR-181a-5p. Exp Anim. 2020;69(1):34–44. https://doi.org/10.1538/expanim.19-0058.
Kim MN, Moon JH, Cho YM. Sodium-glucose cotransporter-2 inhibition reduces cellular senescence in the diabetic kidney by promoting ketone body-induced NRF2 activation. Diabetes Obes Metab. 2021;23(11):2561–71. https://doi.org/10.1111/dom.14503.
Park SH, Farooq MA, Gaertner S, et al. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovasc Diabetol. 2020;19(1):19. https://doi.org/10.1186/s12933-020-00997-7.
Van Linthout S, Seeland U, Riad A, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008;103(4):319–27. https://doi.org/10.1007/s00395-008-0715-2.
Wang SQ, Li D, Yuan Y. Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-beta1/Smad pathway. J Physiol Sci. 2019;69(6):861–73. https://doi.org/10.1007/s12576-019-00696-3.
Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA. alpha11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res. 2012;96(2):265–75. https://doi.org/10.1093/cvr/cvs259.
Pan X, Phanish MK, Baines DL, Dockrell MEC. High glucose-induced Smad3 linker phosphorylation and CCN2 expression are inhibited by dapagliflozin in a diabetic tubule epithelial cell model. Biosci Rep. 2021;41(6) https://doi.org/10.1042/BSR20203947.
Xiao T, Zeng O, Luo J, et al. Effects of hydrogen sulfide on myocardial fibrosis in diabetic rats: changes in matrix metalloproteinases parameters. Biomed Mater Eng. 2015;26(Suppl 1):S2033–9. https://doi.org/10.3233/BME-151508.
Dede E, Liapis D, Davos C, et al. The effects of exercise training on cardiac matrix metalloproteinases activity and cardiac function in mice with diabetic cardiomyopathy. Biochem Biophys Res Commun. 2022;586:8–13. https://doi.org/10.1016/j.bbrc.2021.11.013.
Dawood AF, Alzamil NM, Hewett PW, et al. Metformin protects against diabetic cardiomyopathy: an association between Desmin-Sarcomere injury and the iNOS/mTOR/TIMP-1 fibrosis axis. Biomedicines. 2022;10(5) https://doi.org/10.3390/biomedicines10050984.
Meng L, Uzui H, Guo H, Tada H. Role of SGLT1 in high glucose level-induced MMP-2 expression in human cardiac fibroblasts. Mol Med Rep. 2018;17(5):6887–92. https://doi.org/10.3892/mmr.2018.8688.
Li W, Lou X, Zha Y, et al. Single-cell RNA-seq of heart reveals intercellular communication drivers of myocardial fibrosis in diabetic cardiomyopathy. Elife. 2023:12. https://doi.org/10.7554/eLife.80479.
Saucedo-Orozco H, Voorrips SN, Yurista SR, de Boer RA, Westenbrink BD. SGLT2 Inhibitors and ketone metabolism in heart failure. J Lipid Atheroscler. 2022;11(1):1–19. https://doi.org/10.12997/jla.2022.11.1.1.
Chen S, Coronel R, Hollmann MW, Weber NC, Zuurbier CJ. Direct cardiac effects of SGLT2 inhibitors. Cardiovasc Diabetol. 2022;21(1):45. https://doi.org/10.1186/s12933-022-01480-1.
Da Silva D, Ausina P, Alencar EM, et al. Metformin reverses hexokinase and phosphofructokinase downregulation and intracellular distribution in the heart of diabetic mice. IUBMB Life. 2012;64(9):766–74. https://doi.org/10.1002/iub.1063.
Sousa Fialho MDL, Purnama U, Dennis K, et al. Activation of HIF1alpha rescues the hypoxic response and reverses metabolic dysfunction in the diabetic heart. Diabetes. 2021;70(11):2518–31. https://doi.org/10.2337/db21-0398.
Byrne NJ, Rajasekaran NS, Abel ED, Bugger H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic Biol Med. 2021;169:317–42. https://doi.org/10.1016/j.freeradbiomed.2021.03.046.
Sha W, Hu F, Xi Y, Chu Y, Bu S. Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J Diabetes Res. 2021;2021:9999612. https://doi.org/10.1155/2021/9999612.
Katunga LA, Gudimella P, Efird JT, et al. Obesity in a model of gpx4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Mol Metab. 2015;4(6):493–506. https://doi.org/10.1016/j.molmet.2015.04.001.
Baseler WA, Dabkowski ER, Jagannathan R, et al. Reversal of mitochondrial proteomic loss in type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. Am J Physiol Regul Integr Comp Physiol. 2013;304(7):R553–65. https://doi.org/10.1152/ajpregu.00249.2012.
Feng W, Wang Y, Cai L, Kang YJ. Metallothionein rescues hypoxia-inducible factor-1 transcriptional activity in cardiomyocytes under diabetic conditions. Biochem Biophys Res Commun. 2007;360(1):286–9. https://doi.org/10.1016/j.bbrc.2007.06.057.
Huang S, Wang J, Men H, et al. Cardiac metallothionein overexpression rescues diabetic cardiomyopathy in Akt2-knockout mice. J Cell Mol Med. 2021;25(14):6828–40. https://doi.org/10.1111/jcmm.16687.
Gu J, Yan X, Dai X, et al. Metallothionein preserves Akt2 activity and cardiac function via inhibiting TRB3 in diabetic hearts. Diabetes. 2018;67(3):507–17. https://doi.org/10.2337/db17-0219.
Gu J, Cheng Y, Wu H, et al. Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66(2):529–42. https://doi.org/10.2337/db15-1274.
Kobylecki CJ, Afzal S, Nordestgaard BG. Genetically low antioxidant protection and risk of cardiovascular disease and heart failure in diabetic subjects. EBioMedicine. 2015;2(12):2010–5. https://doi.org/10.1016/j.ebiom.2015.11.026.
Gao YH, Li CX, Shen SM, et al. Hypoxia-inducible factor 1alpha mediates the down-regulation of superoxide dismutase 2 in von Hippel-Lindau deficient renal clear cell carcinoma. Biochem Biophys Res Commun. 2013;435(1):46–51. https://doi.org/10.1016/j.bbrc.2013.04.034.
Bohuslavova R, Kolar F, Sedmera D, et al. Partial deficiency of HIF-1alpha stimulates pathological cardiac changes in streptozotocin-induced diabetic mice. BMC Endocr Disord. 2014;14:11. https://doi.org/10.1186/1472-6823-14-11.
Gao Q, Guan L, Hu S, et al. Study on the mechanism of HIF1a-SOX9 in glucose-induced cardiomyocyte hypertrophy. Biomed Pharmacother. 2015;74:57–62. https://doi.org/10.1016/j.biopha.2015.07.009.
Huang YT, Liu CH, Yang YC, et al. ROS- and HIF1alpha-dependent IGFBP3 upregulation blocks IGF1 survival signaling and thereby mediates high-glucose-induced cardiomyocyte apoptosis. J Cell Physiol. 2019;234(8):13557–70. https://doi.org/10.1002/jcp.28034.
Packer M. Mechanisms leading to differential hypoxia-inducible factor signaling in the diabetic kidney: modulation by SGLT2 inhibitors and hypoxia mimetics. Am J Kidney Dis. 2021;77(2):280–6. https://doi.org/10.1053/j.ajkd.2020.04.016.
Kondo K, Ishigaki Y, Gao J, et al. Bach1 deficiency protects pancreatic beta-cells from oxidative stress injury. Am J Physiol Endocrinol Metab. 2013;305(5):E641–8. https://doi.org/10.1152/ajpendo.00120.2013.
Yan B, Jiao S, Zhang HS, et al. Prolyl hydroxylase domain protein 3 targets Pax2 for destruction. Biochem Biophys Res Commun. 2011;409(2):315–20. https://doi.org/10.1016/j.bbrc.2011.05.012.
Li CL, Liu B, Wang ZY, et al. Salvianolic acid B improves myocardial function in diabetic cardiomyopathy by suppressing IGFBP3. J Mol Cell Cardiol. 2020;139:98–112. https://doi.org/10.1016/j.yjmcc.2020.01.009.
Lai J, Chen F, Chen J, et al. Overexpression of decorin promoted angiogenesis in diabetic cardiomyopathy via IGF1R-AKT-VEGF signaling. Sci Rep. 2017;7:44473. https://doi.org/10.1038/srep44473.
Ramakrishnan S, Anand V, Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol. 2014;9(2):142–60. https://doi.org/10.1007/s11481-014-9531-7.
Lu H, Fedak PW, Dai X, et al. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation. 2006;114(21):2271–9. https://doi.org/10.1161/CIRCULATIONAHA.106.642330.
Wicik Z, Nowak A, Jarosz-Popek J, et al. Characterization of the SGLT2 interaction network and its regulation by SGLT2 inhibitors: a bioinformatic analysis. Front Pharmacol. 2022;13:901340. https://doi.org/10.3389/fphar.2022.901340.
Gupte M, Umbarkar P, Lal H. Mechanistic insights of empagliflozin-mediated cardiac benefits: nearing the starting line : editorial to: "Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes" by N. Hammoudi et al. Cardiovasc Drugs Ther. 2017;31(3):229–32. https://doi.org/10.1007/s10557-017-6741-2.
Min W, Bin ZW, Quan ZB, Hui ZJ, Sheng FG. The signal transduction pathway of PKC/NF-kappa B/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovasc Diabetol. 2009;8:8. https://doi.org/10.1186/1475-2840-8-8.
Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ. Breviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathway. Acta Pharmacol Sin. 2009;30(8):1081–91. https://doi.org/10.1038/aps.2009.95.
Radovits T, Korkmaz S, Matyas C, et al. An altered pattern of myocardial histopathological and molecular changes underlies the different characteristics of type-1 and type-2 diabetic cardiac dysfunction. J Diabetes Res. 2015;2015:728741. https://doi.org/10.1155/2015/728741.
Oh CC, Lee J, D'Souza K, et al. Activator protein-1 and caspase 8 mediate p38alpha MAPK-dependent cardiomyocyte apoptosis induced by palmitic acid. Apoptosis. 2019;24(5-6):395–403. https://doi.org/10.1007/s10495-018-01510-y.
Zhu N, Huang B, Zhu L, Wang Y. Potential mechanisms of triptolide against diabetic cardiomyopathy based on network pharmacology analysis and molecular docking. J Diabetes Res. 2021;2021:9944589. https://doi.org/10.1155/2021/9944589.
Wernig G, Chen SY, Cui L, et al. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA. 2017;114(18):4757–62. https://doi.org/10.1073/pnas.1621375114.
Wrann CD, Rosen ED. New insights into adipocyte-specific leptin gene expression. Adipocyte. 2012;1(3):168–72. https://doi.org/10.4161/adip.20574.
Wrann CD, Eguchi J, Bozec A, et al. FOSL2 promotes leptin gene expression in human and mouse adipocytes. J Clin Invest. 2012;122(3):1010–21. https://doi.org/10.1172/JCI58431.
Li J, Li S, Hu Y, et al. The expression level of mRNA, protein, and DNA methylation status of FOSL2 of Uyghur in XinJiang in type 2 diabetes. J Diabetes Res. 2016;2016:5957404. https://doi.org/10.1155/2016/5957404.
Liang Y, Yuan W, Zhu W, et al. Macrophage migration inhibitory factor promotes expression of GLUT4 glucose transporter through MEF2 and Zac1 in cardiomyocytes. Metabolism. 2015;64(12):1682–93. https://doi.org/10.1016/j.metabol.2015.09.007.
Wang J, Tong C, Yan X, et al. Limiting cardiac ischemic injury by pharmacological augmentation of macrophage migration inhibitory factor-AMP-activated protein kinase signal transduction. Circulation. 2013;128(3):225–36. https://doi.org/10.1161/CIRCULATIONAHA.112.000862.
Zhang D, Golubkov VS, Han W, et al. Identification of Annexin A4 as a hepatopancreas factor involved in liver cell survival. Dev Biol. 2014;395(1):96–110. https://doi.org/10.1016/j.ydbio.2014.08.025.
Monkemann H, De Vriese AS, Blom HJ, et al. Early molecular events in the development of the diabetic cardiomyopathy. Amino Acids. 2002;23(1-3):331–6. https://doi.org/10.1007/s00726-001-0146-y.
Kitada K, Nakano D, Ohsaki H, et al. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications. 2014;28(5):604–11. https://doi.org/10.1016/j.jdiacomp.2014.05.010.
Almeida De Oliveira A, Faustino J, Pedrosa Nunes KJH. Abstract P190: transcriptomic data analysis reveals sex-related differences in the interplay between toll-like receptor 4 and heat-shock protein 70 in the aorta of type 2 diabetic donors. Hypertension. 2019;74(Suppl_1):AP190-AP. https://doi.org/10.1161/hyp.74.suppl_1.P190.
Fiorese CJ, Schulz AM, Lin YF, et al. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol. 2016;26(15):2037–43. https://doi.org/10.1016/j.cub.2016.06.002.
Juliana CA, Yang J, Rozo AV, et al. ATF5 regulates beta-cell survival during stress. Proc Natl Acad Sci USA. 2017;114(6):1341–6. https://doi.org/10.1073/pnas.1620705114.
Pei-Yuan Z, Yu-Wei L, Xiang-Nan Z, et al. Overexpression of Axl reverses endothelial cells dysfunction in high glucose and hypoxia. J Cell Biochem. 2019;120(7):11831–41. https://doi.org/10.1002/jcb.28462.
Wu W, Xu H, Meng Z, et al. Axl is essential for in-vitro angiogenesis induced by vitreous from patients with proliferative diabetic retinopathy. Front Med (Lausanne). 2021;8:787150. https://doi.org/10.3389/fmed.2021.787150.
Su SC, Chiang CF, Hsieh CH, et al. Growth arrest-specific 6 modulates adiponectin expression and insulin resistance in adipose tissue. J Diabetes Investig. 2021;12(4):485–92. https://doi.org/10.1111/jdi.13412.
Arai H, Nagai K, Doi T. Role of growth arrest-specific gene 6 in diabetic nephropathy. Vitam Horm. 2008;78:375–92. https://doi.org/10.1016/S0083-6729(07)00015-5.
Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem. 1996;44(12):1469–79. https://doi.org/10.1177/44.12.8985139.
Burlina S, Banfi C, Brioschi M, et al. Is the placental proteome impaired in well-controlled gestational diabetes? J Mass Spectrom. 2019;54(4):359–65. https://doi.org/10.1002/jms.4336.
Zhang Y, Cui L, Guan G, et al. Matrine suppresses cardiac fibrosis by inhibiting the TGF-beta/Smad pathway in experimental diabetic cardiomyopathy. Mol Med Rep. 2018;17(1):1775–81. https://doi.org/10.3892/mmr.2017.8054.
Malek V, Gaikwad AB. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc Res. 2019;115(2):373–84. https://doi.org/10.1093/cvr/cvy226.
Che H, Wang Y, Li Y, et al. Inhibition of microRNA-150-5p alleviates cardiac inflammation and fibrosis via targeting Smad7 in high glucose-treated cardiac fibroblasts. J Cell Physiol. 2020;235(11):7769–79. https://doi.org/10.1002/jcp.29386.
Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117(6):1450–88. https://doi.org/10.1093/cvr/cvaa324
Wang Y, Yu K, Zhao C, et al. Follistatin attenuates myocardial fibrosis in diabetic cardiomyopathy via the TGF-beta-Smad3 pathway. Front Pharmacol. 2021;12:683335. https://doi.org/10.3389/fphar.2021.683335.
Dong L, Li JC, Hu ZJ, et al. Deletion of Smad3 protects against diabetic myocardiopathy in db/db mice. J Cell Mol Med. 2021;25(10):4860–9. https://doi.org/10.1111/jcmm.16464.
Panchapakesan U, Pegg K, Gross S, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells--renoprotection in diabetic nephropathy? PLoS One. 2013;8(2):e54442. https://doi.org/10.1371/journal.pone.0054442.
Xu H, Qing T, Shen Y, et al. RNA-seq analyses the effect of high-salt diet in hypertension. Gene. 2018;677:245–50. https://doi.org/10.1016/j.gene.2018.07.069.
Gallini R, Lindblom P, Bondjers C, Betsholtz C, Andrae J. PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice. Exp Cell Res. 2016;349(2):282–90. https://doi.org/10.1016/j.yexcr.2016.10.022.
Ye Y, Jia X, Bajaj M, Birnbaum Y. Dapagliflozin attenuates Na(+)/H(+) exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Ther. 2018;32(6):553–8. https://doi.org/10.1007/s10557-018-6837-3.
Acknowledgements
We would like to thank Hsiu-Chiung Yang and Stefano Bartesaghi for the administrative management of the collaboration. We would like to thank the NGS Core facility of the University of Texas for the generation and pre-processing of the RNA-sequencing experiment.
Funding
This research has been funded by AstraZeneca. The first author is an AstraZeneca employee and had direct involvement in the data analysis, interpretation, and planning of the follow-up experiments.
Author information
Authors and Affiliations
Contributions
M.R.—computational analysis of RNA-seq data, hypotheses for follow-up experiments, and first manuscript draft. Y.Y. and Y.B.—study design, in vivo experiments, RT-PCR, and protein quantification. R.Y—literature search, data analysis, and editing the manuscript. All authors contributed to data interpretation, revised the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
M.R. is an employee of AstraZeneca and may hold company shares/stocks. Y.Y. and Y. B. received the research grant from AstraZeneca.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 6130 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ryaboshapkina, M., Ye, R., Ye, Y. et al. Effects of Dapagliflozin on Myocardial Gene Expression in BTBR Mice with Type 2 Diabetes. Cardiovasc Drugs Ther 39, 43–61 (2025). https://doi.org/10.1007/s10557-023-07517-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-023-07517-1