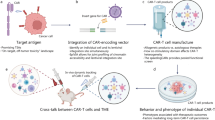
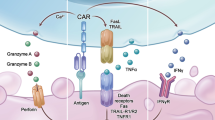
Abstract
Cellular and targeted immunotherapies have revolutionized cancer treatments in the last several decades. Successful cellular therapies require both effective and durable cytotoxic activity from the immune cells as well as an accessible and susceptible response from targeted cancer cells. Correlative studies from clinical trials as well as real-world data from FDA-approved therapies have revealed invaluable insights about immune cell factors and cancer cell factors that impact rates of response and relapse to cellular therapies. This review focuses on the flagship cellular therapy of engineered chimeric antigen receptor T-cells (CAR-T cells). Within the CAR-T cell compartment, we discuss discoveries about T-cell phenotype, transcriptome, epigenetics, cytokine signaling, and metabolism that inform the cell manufacturing process to produce the most effective and durable CAR-T cells. Within the cancer cell compartment, we discuss mechanisms of resistance and relapse caused by mutations, alternative splicing, post-transcriptional modifications, and cellular reprogramming. Continued correlative and mechanistic studies are required to help us further optimize cellular therapies in a variety of malignancies.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
No datasets were generated or analyzed during the current study.
References
Raje, N., Berdeja, J., Lin, Y., Siegel, D., Jagannath, S., Madduri, D., …, Kochenderfer, J. N. (2019). Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. New England Journal of Medicine, 380(18), 1726–1737. https://doi.org/10.1056/nejmoa1817226
Schuster, S. J., Bishop, M. R., Tam, C. S., Waller, E. K., Borchmann, P., McGuirk, J. P., …, Maziarz, R. T. (2019). Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. New England Journal of Medicine, 380(1), 45–56. https://doi.org/10.1056/NEJMOA1804980/SUPPL_FILE/NEJMOA1804980_DISCLOSURES.PDF
Neelapu, S. S., Locke, F. L., Bartlett, N. L., Lekakis, L. J., Miklos, D. B., Jacobson, C. A., …, Go, W. Y. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377(26), 2531–2544. https://doi.org/10.1056/NEJMOA1707447/SUPPL_FILE/NEJMOA1707447_DISCLOSURES.PDF
Lee, D. W., Kochenderfer, J. N., Stetler-Stevenson, M., Cui, Y. K., Delbrook, C., Feldman, S. A., …, Mackall, C. L. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet (London, England), 385(9967), 517. https://doi.org/10.1016/S0140-6736(14)61403-3
Maude, S. L., Laetsch, T. W., Buechner, J., Rives, S., Boyer, M., Bittencourt, H., …, Grupp, S. A. (2018). Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New England Journal of Medicine, 378(5), 439–448. https://doi.org/10.1056/NEJMOA1709866/SUPPL_FILE/NEJMOA1709866_DISCLOSURES.PDF
Pulsipher, M. A., Han, X., Maude, S. L., Laetsch, T. W., Qayed, M., Rives, S., …, Grupp, S. A. (2022). Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discovery, 3(1), 66–81. https://doi.org/10.1158/2643-3230.BCD-21-0095
Gardner, R. A., Finney, O., Annesley, C., Brakke, H., Summers, C., Leger, K., …, Jensen, M. C. (2017). Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood, 129(25), 3322. https://doi.org/10.1182/BLOOD-2017-02-769208
Kochenderfer, J. N., Somerville, R. P. T., Lu, T., Yang, J. C., Sherry, R. M., Feldman, S. A., …, Rosenberg, S. A. (2017). Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Molecular Therapy, 25(10), 2245–2253. https://doi.org/10.1016/j.ymthe.2017.07.004
Park, J. H., Rivière, I., Gonen, M., Wang, X., Sénéchal, B., Curran, K. J., …, Sadelain, M. (2018). Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. New England Journal of Medicine, 378(5), 449–459. https://doi.org/10.1056/NEJMOA1709919/SUPPL_FILE/NEJMOA1709919_DISCLOSURES.PDF
Shalabi, H., Delbrook, C., Stetler-Stevenson, M., Yuan, C., Steinberg, S. M., Yates, B., …, Shah, N. N. (2018). Chimeric antigen receptor T-cell (CAR-T) therapy can render patients with ALL into PCR-negative remission and can be an effective bridge to transplant (HCT). Biology of Blood and Marrow Transplantation, 24(3), S25–S26. https://doi.org/10.1016/j.bbmt.2017.12.018
Maude, S. L., Frey, N., Shaw, P. A., Aplenc, R., Barrett, D. M., Bunin, N. J., …, Grupp, S. A. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine, 371(16), 1507. https://doi.org/10.1056/NEJMOA1407222
Mount, C. W., & Gonzalez Castro, L. N. (2022). Advances in chimeric antigen receptor (CAR) T-cell therapies for the treatment of primary brain tumors. Antibodies, 11(2). https://doi.org/10.3390/ANTIB11020031
Majzner, R. G., Ramakrishna, S., Yeom, K. W., Patel, S., Chinnasamy, H., Schultz, L. M., …, Monje, M. (2022). GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature, 603(7903), 934–941. https://doi.org/10.1038/s41586-022-04489-4
Cummins, K. D., & Gill, S. (2019). Will CAR T cell therapy have a role in AML? Promises and pitfalls. Seminars in Hematology, 56(2), 155–163. https://doi.org/10.1053/j.seminhematol.2018.08.008
Feucht, J., Sun, J., Eyquem, J., Ho, Y. J., Zhao, Z., Leibold, J., …, Sadelain, M. (2018). Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nature Medicine 2018 25:1, 25(1), 82–88. https://doi.org/10.1038/S41591-018-0290-5
Hamieh, M., Mansilla-Soto, J., Rivière, I., & Sadelain, M. (2023). Programming CAR T cell tumor recognition: Tuned antigen sensing and logic gating. Cancer Discovery, 13(4), 829. https://doi.org/10.1158/2159-8290.CD-23-0101
Haubner, S., Mansilla-Soto, J., Nataraj, S., Kogel, F., Chang, Q., de Stanchina, E., …, Sadelain, M. (2023). Cooperative CAR targeting to selectively eliminate AML and minimize escape. Cancer Cell, 41(11), 1871–1891.e6. https://doi.org/10.1016/J.CCELL.2023.09.010
Tousley, A. M., Rotiroti, M. C., Labanieh, L., Rysavy, L. W., Kim, W. J., Lareau, C., …, Majzner, R. G. (2023). Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 2023 615:7952, 615(7952), 507–516. https://doi.org/10.1038/S41586-023-05778-2
Labanieh, L., & Mackall, C. L. (2023). CAR immune cells: Design principles, resistance and the next generation. Nature 2023 614:7949, 614(7949), 635–648. https://doi.org/10.1038/s41586-023-05707-3
Melenhorst, J. J., Chen, G. M., Wang, M., Porter, D. L., Chen, C., Collins, M. K. A., …, June, C. H. (2022). Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature, 602(7897), 503–509. https://doi.org/10.1038/s41586-021-04390-6
Wilson, T. L., Kim, H., Chou, C. H., Langfitt, D., Mettelman, R. C., Minervina, A. A., …, Thomas, P. G. (2022). Common trajectories of highly effective CD19-specific CAR T cells identified by endogenous T-cell receptor lineages. Cancer Discovery, 12(9), 2098–2119. https://doi.org/10.1158/2159-8290.CD-21-1508
Anderson, N. D., Birch, J., Accogli, T., Criado, I., Khabirova, E., Parks, C., …, Ghorashian, S. (2023). Transcriptional signatures associated with persisting CD19 CAR-T cells in children with leukemia. Nature Medicine, 29(7), 1700–1709. https://doi.org/10.1038/s41591-023-02415-3
Yu, X., Harden, K., Gonzalez, L. C., Francesco, M., Chiang, E., Irving, B., …, Grogan, J. L. (2008). The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nature Immunology 2008 10:1, 10(1), 48–57. https://doi.org/10.1038/ni.1674
Good, Z., Spiegel, J. Y., Sahaf, B., Malipatlolla, M. B., Ehlinger, Z. J., Kurra, S., …, Mackall, C. L. (2022). Post-infusion CAR TReg cells identify patients resistant to CD19-CAR therapy. Nature Medicine, 28(9), 1860–1871. https://doi.org/10.1038/s41591-022-01960-7
Haradhvala, N. J., Leick, M. B., Maurer, K., Gohil, S. H., Larson, R. C., Yao, N., …, Maus, M. V. (2022). Distinct cellular dynamics associated with response to CAR-T therapy for refractory B-cell lymphoma. Nature Medicine, 28(9), 1848. https://doi.org/10.1038/S41591-022-01959-0
Seo, H., Chen, J., González-Avalos, E., Samaniego-Castruita, D., Das, A., Wang, Y. H., …, Rao, A. (2019). TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proceedings of the National Academy of Sciences of the United States of America, 116(25), 12410–12415. https://doi.org/10.1073/PNAS.1905675116/SUPPL_FILE/PNAS.1905675116.SAPP.PDF
Chen, J., López-Moyado, I. F., Seo, H., Lio, C. W. J., Hempleman, L. J., Sekiya, T., …, Rao, A. (2019). NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019 567:7749, 567(7749), 530–534. https://doi.org/10.1038/S41586-019-0985-X
Lynn, R. C., Weber, E. W., Sotillo, E., Gennert, D., Xu, P., Good, Z., …, Mackall, C. L. (2019). c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature, 576(7786), 293–300. https://doi.org/10.1038/s41586-019-1805-z
Zhang, X., Zhang, C., Qiao, M., Cheng, C., Tang, N., Lu, S., …, Wang, H. (2022). Depletion of BATF in CAR-T cells enhances antitumor activity by inducing resistance against exhaustion and formation of central memory cells. Cancer Cell, 40(11), 1407–1422.e7. https://doi.org/10.1016/J.CCELL.2022.09.013
McCutcheon, S. R., Swartz, A. M., Brown, M. C., Barrera, A., McRoberts Amador, C., Siklenka, K., …, Gersbach, C. A. (2023). Transcriptional and epigenetic regulators of human CD8+ T cell function identified through orthogonal CRISPR screens. Nature Genetics 2023 55:12, 55(12), 2211–2223. https://doi.org/10.1038/S41588-023-01554-0
Seo, H., González-Avalos, E., Zhang, W., Ramchandani, P., Yang, C., Lio, C. W. J., …, Hogan, P. G. (2021). BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells. Nature Immunology 2021 22:8, 22(8), 983–995. https://doi.org/10.1038/S41590-021-00964-8
Doan, A. E., Mueller, K. P., Chen, A. Y., Rouin, G. T., Chen, Y., Daniel, B., …, Weber, E. W. (2024). FOXO1 is a master regulator of memory programming in CAR T cells. Nature. https://doi.org/10.1038/s41586-024-07300-8
Tang, J., Sheng, J., Zhang, Q., Ji, Y., Wang, X., Zhang, J., …, Liang, T. (2023). Runx3-overexpression cooperates with ex vivo AKT inhibition to generate receptor-engineered T cells with better persistence, tumor-residency, and antitumor ability. Journal for ImmunoTherapy of Cancer, 11(2), e006119. https://doi.org/10.1136/JITC-2022-006119
Chen, Z., Arai, E., Khan, O., Zhang, Z., Ngiow, S. F., He, Y., …, Shi, J. (2021). In vivo CD8+ T cell CRISPR screening reveals control by Fli1 in infection and cancer. Cell, 184(5), 1262–1280.e22. https://doi.org/10.1016/J.CELL.2021.02.019
Fraietta, J. A., Nobles, C. L., Sammons, M. A., Lundh, S., Carty, S. A., Reich, T. J., …, Melenhorst, J. J. (2018). Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature, 558(7709), 307–312. https://doi.org/10.1038/s41586-018-0178-z
Jain, N., Zhao, Z., Feucht, J., Koche, R., Iyer, A., Dobrin, A., …, Sadelain, M. (2023). TET2 guards against unchecked BATF3-induced CAR T cell expansion. Nature 2023 615:7951, 615(7951), 315–322. https://doi.org/10.1038/S41586-022-05692-Z
Belk, J. A., Yao, W., Ly, N., Freitas, K. A., Chen, Y. T., Shi, Q., …, Satpathy, A. T. (2022). Genome-wide CRISPR screens of T cell exhaustion identify chromatin remodeling factors that limit T cell persistence. Cancer Cell, 40(7), 768–786.e7. https://doi.org/10.1016/j.ccell.2022.06.001
Prinzing, B., Zebley, C. C., Petersen, C. T., Fan, Y., Anido, A. A., Yi, Z., …, Krenciute, G. (2021). Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Science Translational Medicine, 13(620), eabh0272. https://doi.org/10.1126/SCITRANSLMED.ABH0272
Jain, N., Zhao, Z., Koche, R. P., Antelope, C., Gozlan, Y., Montalbano, A., …, Sadelain, M. (2024). Disruption of SUV39H1-mediated H3K9 methylation sustains CAR t-cell function. Cancer Discovery, 14(1), 142–157. https://doi.org/10.1158/2159-8290.CD-22-1319/729924/AM/DISRUPTION-OF-SUV39H1-MEDIATED-H3K9-METHYLATION
López-Cobo, S., Fuentealba, J. R., Gueguen, P., Bonté, P. E., Tsalkitzi, K., Chacón, I., …, Amigorena, S. (2024). SUV39H1 ablation enhances long-term CAR T function in solid tumors. Cancer Discovery, 14(1), 120–141. https://doi.org/10.1158/2159-8290.CD-22-1350/729925/AM/SUV39H1-ABLATION-ENHANCES-LONG-TERM-CAR-T-FUNCTION
Weber, E. W., Parker, K. R., Sotillo, E., Lynn, R. C., Anbunathan, H., Lattin, J., …, Mackall, C. L. (2021). Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science, 372(6537). https://doi.org/10.1126/SCIENCE.ABA1786/SUPPL_FILE/ABA1786_WEBER_SM.PDF
Zhang, H., Hu, Y., Shao, M., Teng, X., Jiang, P., Wang, X., …, Huang, H. (2021). Dasatinib enhances anti-leukemia efficacy of chimeric antigen receptor T cells by inhibiting cell differentiation and exhaustion. Journal of Hematology and Oncology, 14(1), 1–6. https://doi.org/10.1186/S13045-021-01117-Y/FIGURES/2
Mestermann, K., Giavridis, T., Weber, J., Rydzek, J., Frenz, S., Nerreter, T., …, Hudecek, M. (2019). The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR-T cells. Science Translational Medicine, 11(499). https://doi.org/10.1126/SCITRANSLMED.AAU5907
Xu, Y., Zhang, M., Ramos, C. A., Durett, A., Liu, E., Dakhova, O., …, Dotti, G. (2014). Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood, 123(24), 3750–3759. https://doi.org/10.1182/blood-2014-01-552174
Bell, M., & Gottschalk, S. (2021). Engineered cytokine signaling to improve CAR T cell effector function. Frontiers in Immunology, 12, 684642. https://doi.org/10.3389/FIMMU.2021.684642/BIBTEX
Markley, J. C., & Sadelain, M. (2010). IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell–mediated rejection of systemic lymphoma in immunodeficient mice. Blood, 115(17), 3508–3519. https://doi.org/10.1182/BLOOD-2009-09-241398
Del Galy, A. S., Menegatti, S., Fuentealba, J., Lucibello, F., Perrin, L., Helft, J., …, Menger, L. (2021). In vivo genome-wide CRISPR screens identify SOCS1 as intrinsic checkpoint of CD4+T H 1 cell response. Science Immunology, 6(66), 8219. https://doi.org/10.1126/SCIIMMUNOL.ABE8219/SUPPL_FILE/SCIIMMUNOL.ABE8219_TABLES_S1_TO_S5.ZIP
Wenes, M., Jaccard, A., Wyss, T., Maldonado-Pérez, N., Teoh, S. T., Lepez, A., …, Romero, P. (2022). The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function. Cell Metabolism, 34(5), 731–746.e9. https://doi.org/10.1016/j.cmet.2022.03.013
Jaccard, A., Wyss, T., Maldonado-Pérez, N., Rath, J. A., Bevilacqua, A., Peng, J. J., …, Wenes, M. (2023). Reductive carboxylation epigenetically instructs T cell differentiation. Nature 2023 621:7980, 621(7980), 849–856. https://doi.org/10.1038/S41586-023-06546-Y
Si, X., Shao, M., Teng, X., Huang, Y., Meng, Y., Wu, L., …, Huang, H. (2024). Mitochondrial isocitrate dehydrogenase impedes CAR T cell function by restraining antioxidant metabolism and histone acetylation. Cell Metabolism, 36(1), 176–192.e10. https://doi.org/10.1016/J.CMET.2023.12.010/ATTACHMENT/92E3FA39-8112-4573-A1F5-8D938B787C95/MMC3.PDF
Klysz, D. D., Fowler, C., Malipatlolla, M., Stuani, L., Freitas, K. A., Chen, Y., …, Mackall, C. L. (2024). Inosine induces stemness features in CAR-T cells and enhances potency. Cancer Cell, 42(2), 266–282.e8. https://doi.org/10.1016/j.ccell.2024.01.002
Linnemann, C., Schildberg, F. A., Schurich, A., Diehl, L., Hegenbarth, S. I., Endl, E., …, Knolle, P. A. (2009). Adenosine regulates CD8 T-cell priming by inhibition of membrane-proximal T-cell receptor signalling. Immunology, 128(1pt2), e728–e737. https://doi.org/10.1111/J.1365-2567.2009.03075.X
Vormittag, P., Gunn, R., Ghorashian, S., & Veraitch, F. S. (2018). A guide to manufacturing CAR T cell therapies. Current Opinion in Biotechnology, 53, 164–181. https://doi.org/10.1016/J.COPBIO.2018.01.025
Ghassemi, S., Nunez-Cruz, S., O’Connor, R. S., Fraietta, J. A., Patel, P. R., Scholler, J., …, Milone, M. C. (2018). Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunology Research, 6(9), 1100–1109. https://doi.org/10.1158/2326-6066.CIR-17-0405/470662/AM/REDUCING-EX-VIVO-CULTURE-IMPROVES-THE-ANTI
Laetsch, T. W., Maude, S. L., Rives, S., Hiramatsu, H., Bittencourt, H., Bader, P., …, Grupp, S. A. (2023). Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. Journal of Clinical Oncology, 41(9), 1664. https://doi.org/10.1200/JCO.22.00642
Fry, T. J., Shah, N. N., Orentas, R. J., Stetler-Stevenson, M., Yuan, C. M., Ramakrishna, S., …, Mackall, C. L. (2018). CD22-CAR T cells induce remissions in CD19-CAR naïve and resistant B-ALL. Nature medicine, 24(1), 20. https://doi.org/10.1038/NM.4441
Faruqi, A. J., Ligon, J. A., Borgman, P., Steinberg, S. M., Foley, T., Little, L., …, Shah, N. N. (2022). The impact of race, ethnicity, and obesity on CAR T-cell therapy outcomes. Blood Advances, 6(23), 6040–6050. https://doi.org/10.1182/BLOODADVANCES.2022007676
Lamble, A. J., Myers, R. M., Taraseviciute, A., John, S., Yates, B., Steinberg, S. M., …, Shah, N. N. (2023). Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Advances, 7(4), 575–585. https://doi.org/10.1182/BLOODADVANCES.2022007423
Majzner, R. G., & Mackall, C. L. (2019). Clinical lessons learned from the first leg of the CAR T cell journey. Nature Medicine, 25(9), 1341–1355. https://doi.org/10.1038/s41591-019-0564-6
Schultz, L. M., Baggott, C., Prabhu, S., Pacenta, H. L., Phillips, C. L., Rossoff, J., …, Laetsch, T. W. (2022). Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: A pediatric real-world chimeric antigen receptor consortium report. Journal of Clinical Oncology, 40(9), 945. https://doi.org/10.1200/JCO.20.03585
Pulsipher, M. A., Han, X., Maude, S. L., Laetsch, T. W., Qayed, M., Rives, S., …, Grupp, S. A. (2022). Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discovery, 3(1), 66–81. https://doi.org/10.1158/2643-3230.BCD-21-0095/674665/P/NEXT-GENERATION-SEQUENCING-OF-MINIMAL-RESIDUAL
Myers, R. M., Taraseviciute, A., Steinberg, S. M., Lamble, A. J., Sheppard, J., Yates, B., …, Shah, N. N. (2022). Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. Journal of Clinical Oncology, 40(9), 932. https://doi.org/10.1200/JCO.21.01405
Dourthe, M. E., Rabian, F., Yakouben, K., Chevillon, F., Cabannes-Hamy, A., Méchinaud, F., …, Baruchel, A. (2021). Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia 2021 35:12, 35(12), 3383–3393. https://doi.org/10.1038/s41375-021-01281-7
Turtle, C. J., Hanafi, L. A., Berger, C., Gooley, T. A., Cherian, S., Hudecek, M., …, Maloney, D. G. (2016). CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. The Journal of Clinical Investigation, 126(6), 2123. https://doi.org/10.1172/JCI85309
Holland, E. M., Molina, J. C., Dede, K., Moyer, D., Zhou, T., Yuan, C. M., …, Shah, N. (2022). Efficacy of second CAR-T (CART2) infusion limited by poor CART expansion and antigen modulation. Journal for Immunotherapy of Cancer, 10, 4483. https://doi.org/10.1136/jitc-2021-004483
Turtle, C. J., Hanafi, L. A., Berger, C., Hudecek, M., Pender, B., Robinson, E., …, Maloney, D. G. (2016). Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Science Translational Medicine, 8(355). https://doi.org/10.1126/SCITRANSLMED.AAF8621/SUPPL_FILE/8-355RA116_SM.PDF
Mejstríková, E., Hrusak, O., Borowitz, M. J., Whitlock, J. A., Brethon, B., Trippett, T. M., …, Locatelli, F. (2017). CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer Journal, 7(12), 659. https://doi.org/10.1038/S41408-017-0023-X
Bhojwani, D., Sposto, R., Shah, N. N., Rodriguez, V., Yuan, C., Stetler-Stevenson, M., …, Rheingold, S. R. (2019). Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia, 33(4), 884. https://doi.org/10.1038/S41375-018-0265-Z
Sotillo, E., Barrett, D. M., Black, K. L., Bagashev, A., Oldridge, D., Wu, G., …, Thomas-Tikhonenko, A. (2015). Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discovery, 5(12), 1282–1295. https://doi.org/10.1158/2159-8290.CD-15-1020/43172/P/CONVERGENCE-OF-ACQUIRED-MUTATIONS-AND-ALTERNATIVE
Asnani, M., Hayer, K. E., Naqvi, A. S., Zheng, S., Yang, S. Y., Oldridge, D., …, Thomas-Tikhonenko, A. (2019). Retention of CD19 intron 2 contributes to CART-19 resistance in leukemias with subclonal frameshift mutations in CD19. Leukemia 2019 34:4, 34(4), 1202–1207. https://doi.org/10.1038/s41375-019-0580-z
Fischer, J., Paret, C., El Malki, K., Alt, F., Wingerter, A., Neu, M. A., …, Faber, J. (2017). CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. Journal of Immunotherapy (Hagerstown, Md. : 1997), 40(5), 187. https://doi.org/10.1097/CJI.0000000000000169
Bagashev, A., Sotillo, E., Tang, C.-H. A., Black, K. L., Perazzelli, J., Seeholzer, S. H., …, Thomas-Tikhonenko, A. (2018). CD19 alterations emerging after CD19-directed immunotherapy cause retention of the misfolded protein in the endoplasmic reticulum. Molecular and Cellular Biology, 38(21). https://doi.org/10.1128/MCB.00383-18
Orlando, E. J., Han, X., Tribouley, C., Wood, P. A., Leary, R. J., Riester, M., …, Winckler, W. (2018). Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nature Medicine 2018 24:10, 24(10), 1504–1506. https://doi.org/10.1038/s41591-018-0146-z
Ghobadi, A., Landmann, J. H., Carter, A., Cooper, M. L., Selli, M. E., Chang, J., …, Singh, N. (2022). Discovery of a novel genomic alteration that renders leukemic cells resistant to CD19-targeted immunotherapies. Blood Advances, 6(20), 5634–5640. https://doi.org/10.1182/BLOODADVANCES.2022007705
Fischer, J., Paret, C., El Malki, K., Alt, F., Wingerter, A., Neu, M. A., …, Faber, J. (2017). CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. Journal of Immunotherapy, 40(5), 187–195. https://doi.org/10.1097/CJI.0000000000000169
Rabilloud, T., Potier, D., Pankaew, S., Nozais, M., Loosveld, M., & Payet-Bornet, D. (2021). Single-cell profiling identifies pre-existing CD19-negative subclones in a B-ALL patient with CD19-negative relapse after CAR-T therapy. Nature Communications 2021 12, 12(1), 1–7. https://doi.org/10.1038/s41467-021-21168-6
Bueno, C., Barrera, S., Bataller, A., Ortiz-Maldonado, V., Elliot, N., O’Byrne, S., …, Menendez, P. (2022). CD34+CD19−CD22+ B-cell progenitors may underlie phenotypic escape in patients treated with CD19-directed therapies. Blood, 140(1), 38–44. https://doi.org/10.1182/BLOOD.2021014840
Heard, A., Landmann, J. H., Hansen, A. R., Papadopolou, A., Hsu, Y. S., Selli, M. E., …, Singh, N. (2022). Antigen glycosylation regulates efficacy of CAR T cells targeting CD19. Nature Communications 2022 13:1, 13(1), 1–11. https://doi.org/10.1038/s41467-022-31035-7
Braig, F., Brandt, A., Goebeler, M., Tony, H. P., Kurze, A. K., Nollau, P., …, Binder, M. (2017). Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood, 129(1), 100–104. https://doi.org/10.1182/BLOOD-2016-05-718395
Hamieh, M., Dobrin, A., Cabriolu, A., van der Stegen, S. J. C., Giavridis, T., Mansilla-Soto, J., …, Sadelain, M. (2019). CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019 568:7750, 568(7750), 112–116. https://doi.org/10.1038/s41586-019-1054-1
Fry, T. J., Shah, N. N., Orentas, R. J., Stetler-Stevenson, M., Yuan, C. M., Ramakrishna, S., …, Mackall, C. L. (2017). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nature Medicine 2017 24:1, 24(1), 20–28. https://doi.org/10.1038/nm.4441
Ramakrishna, S., Highfill, S. L., Walsh, Z., Nguyen, S. M., Lei, H., Shern, J. F., …, Fry, T. J. (2019). Modulation of target antigen density improves CAR T-cell functionality and persistence. Clinical Cancer Research, 25(17), 5329–5341. https://doi.org/10.1158/1078-0432.CCR-18-3784/74543/AM/MODULATION-OF-TARGET-ANTIGEN-DENSITY-IMPROVES-CAR
Jacoby, E., Nguyen, S. M., Fountaine, T. J., Welp, K., Gryder, B., Qin, H., …, Fry, T. J. (2016). CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nature Communications. https://doi.org/10.1038/ncomms12320
Lee, B. J., Griffin, S. P., Doh, J., Chan, A., O’Brien, S., Jeyakumar, D., …, Kongtim, P. (2022). CD19-directed immunotherapy use in KMT2A-rearranged acute leukemia: A case report and literature review of increased lymphoid to myeloid lineage switch. American Journal of Hematology, 97(12), E439–E443. https://doi.org/10.1002/AJH.26713
Fournier, E., Inchiappa, L., Delattre, C., Pignon, J. M., Danicourt, F., Bemba, M., …, Duployez, N. (2019). Increased risk of adverse acute myeloid leukemia after anti-CD19-targeted immunotherapies in KMT2A-rearranged acute lymphoblastic leukemia: A case report and review of the literature. Leukemia & Lymphoma, 60(7), 1827–1830. https://doi.org/10.1080/10428194.2018.1562185
Gardner, R., Wu, D., Cherian, S., Fang, M., Hanafi, L. A., Finney, O., …, Turtle, C. J. (2016). Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood, 127(20), 2406. https://doi.org/10.1182/BLOOD-2015-08-665547
Leahy, A. B., Devine, K. J., Li, Y., Liu, H., Myers, R., DiNofia, A., …, Maude, S. L. (2022). Impact of high-risk cytogenetics on outcomes for children and young adults receiving CD19-directed CAR T-cell therapy. Blood, 139(14), 2173–2185. https://doi.org/10.1182/BLOOD.2021012727
Singh, N., Lee, Y. G., Shestova, O., Ravikumar, P., Hayer, K. E., Hong, S. J., …, Ruella, M. (2020). Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T cell dysfunction. Cancer Discovery, 10(4), 552. https://doi.org/10.1158/2159-8290.CD-19-0813
Dufva, O., Koski, J., Maliniemi, P., Ianevski, A., Klievink, J., Leitner, J., …, Mustjoki, S. (2020). Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood, 135(9), 597–609. https://doi.org/10.1182/BLOOD.2019002121
Upadhyay, R., Boiarsky, J. A., Pantsulaia, G., Svensson-Arvelund, J., Lin, M. J., Wroblewska, A., …, Brody, J. D. (2021). A critical role for fas-mediated off-target tumor killing in T cell immunotherapy. Cancer Discovery, 11(3), 599. https://doi.org/10.1158/2159-8290.CD-20-0756
Younes, S., Zhao, S., Bharadwaj, S., Mosquera, A. P., Libert, D., Johnsrud, A., …, Natkunam, Y. (2023). Detection of aberrant CD58 expression in a wide spectrum of lymphoma subtypes: Implications for treatment resistance. Modern Pathology, 36(10), 100256. https://doi.org/10.1016/J.MODPAT.2023.100256
Yan, X., Chen, D., Ma, X., Wang, Y., Guo, Y., Wei, J., …, Han, W. (2022). CD58 loss in tumor cells confers functional impairment of CAR T cells. Blood Advances, 6(22), 5844–5856. https://doi.org/10.1182/BLOODADVANCES.2022007891
Li, Y., Moriyama, T., Yoshimura, S., Zhao, X., Li, Z., Yang, X., …, Yang, J. J. (2022). PAX5 epigenetically orchestrates CD58 transcription and modulates blinatumomab response in acute lymphoblastic leukemia. Science Advances, 8(50). https://doi.org/10.1126/SCIADV.ADD6403/SUPPL_FILE/SCIADV.ADD6403_SM.PDF
Shouval, R., Alarcon Tomas, A., Fein, J. A., Flynn, J. R., Markovits, E., Mayer, S., …, Lia Palomba, M. (2021). Impact of TP53 genomic alterations in large B-cell lymphoma treated with CD19-chimeric antigen receptor T-cell Therapy. Journal of Clinical Oncology, 40, 369–381. https://doi.org/10.1200/JCO.21
Pan, J., Tan, Y., Deng, B., Tong, C., Hua, L., Ling, Z., …, Feng, X. (2020). Frequent occurrence of CD19-negative relapse after CD19 CAR T and consolidation therapy in 14 TP53-mutated r/r B-ALL children. Leukemia, 34(12), 3382–3387. https://doi.org/10.1038/s41375-020-0831-z
Shah, N. N., & Fry, T. J. (2019). Mechanisms of resistance to CAR T cell therapy. Nature Reviews. Clinical Oncology, 16(6), 372. https://doi.org/10.1038/S41571-019-0184-6
Brown, C. E., Hibbard, J. C., Alizadeh, D., Blanchard, M. S., Natri, H. M., Wang, D., …, Badie, B. (2024). Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: A phase 1 trial. Nature Medicine. https://doi.org/10.1038/s41591-024-02875-1
Bagley, S. J., Logun, M., Fraietta, J. A., Wang, X., Desai, A. S., Bagley, L. J., …, O’Rourke, D. M. (2024). Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: Phase 1 trial interim results. Nature Medicine. https://doi.org/10.1038/s41591-024-02893-z
O’Rourke, D. M., Nasrallah, M. P., Desai, A., Melenhorst, J. J., Mansfield, K., Morrissette, J. J. D., …, Maus, M. V. (2017). A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Science Translational Medicine, 9(399). https://doi.org/10.1126/SCITRANSLMED.AAA0984
Ahmed, N., Brawley, V., Hegde, M., Bielamowicz, K., Kalra, M., Landi, D., …, Gottschalk, S. (2017). HER2-specific chimeric antigen receptor–modified virus-specific T cells for progressive glioblastoma: A phase 1 dose-escalation trial. JAMA Oncology, 3(8), 1094–1101. https://doi.org/10.1001/JAMAONCOL.2017.0184
Mount, C. W., Majzner, R. G., Sundaresh, S., Arnold, E. P., Kadapakkam, M., Haile, S., …, Mackall, C. L. (2018). Potent antitumor efficacy of anti-GD2 CAR T-cells in H3K27M+ diffuse midline gliomas. Nature Medicine, 24(5), 572. https://doi.org/10.1038/S41591-018-0006-X
Tang, X., Zhao, S., Zhang, Y., Wang, Y., Zhang, Z., Yang, M., …, Zhou, L. (2019). B7-H3 as a novel CAR-T therapeutic target for glioblastoma. Molecular Therapy Oncolytics, 14, 279–287. https://doi.org/10.1016/J.OMTO.2019.07.002
Tang, X., Wang, Y., Huang, J., Zhang, Z., Liu, F., Xu, J., …, Zhou, L. (2021). Administration of B7-H3 targeted chimeric antigen receptor-T cells induce regression of glioblastoma. Signal Transduction and Targeted Therapy, 6(1). https://doi.org/10.1038/S41392-021-00505-7
Majzner, R. G., Theruvath, J. L., Nellan, A., Heitzeneder, S., Cui, Y., Mount, C. W., …, Mackall, C. L. (2019). CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research, 25(8), 2560. https://doi.org/10.1158/1078-0432.CCR-18-0432
Vitanza, N. A., Wilson, A. L., Huang, W., Seidel, K., Brown, C., Gustafson, J. A., …, Park, J. R. (2023). Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: Preliminary first-in-human bioactivity and safety. Cancer Discovery, 13(1), 114–131. https://doi.org/10.1158/2159-8290.cd-22-0750
Larson, R. C., Kann, M. C., Bailey, S. R., Haradhvala, N. J., Llopis, P. M., Bouffard, A. A., …, Maus, M. V. (2022). CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature, 604(7906), 563–570. https://doi.org/10.1038/s41586-022-04585-5
Del Bufalo, F., De Angelis, B., Caruana, I., Del Baldo, G., De Ioris, M. A., Serra, A., …, Locatelli, F. (2023). GD2-CART01 for relapsed or refractory high-risk neuroblastoma. New England Journal of Medicine, 388(14), 1284–1295. https://doi.org/10.1056/nejmoa2210859
Kaczanowska, S., Murty, T., Alimadadi, A., Contreras, C. F., Duault, C., Subrahmanyam, P. B., …, Kaplan, R. N. (2024). Immune determinants of CAR-T cell expansion in solid tumor patients receiving GD2 CAR-T cell therapy. Cancer Cell, 42(1), 35–51.e8. https://doi.org/10.1016/J.CCELL.2023.11.011
Funding
CY is supported by R38HL143615 from the National Institutes of Health. KLD is supported as the Anne T. and Robert M. Bass Endowed Faculty Scholar in Pediatric Cancer and Blood Diseases through the Stanford Maternal and Child Health Institute.
Author information
Authors and Affiliations
Contributions
CY and KLD wrote and edited the manuscript text. CY prepared Fig. 1. All authors reviewed the manuscript and prepared for submission.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, C.D., Davis, K.L. Correlative studies reveal factors contributing to successful CAR-T cell therapies in cancer. Cancer Metastasis Rev 44, 15 (2025). https://doi.org/10.1007/s10555-024-10232-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10555-024-10232-4
Keywords
Profiles
- Catherine D. Yao View author profile
- Kara L. Davis View author profile