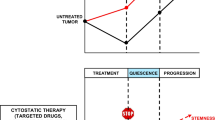
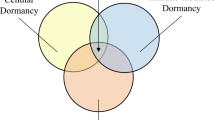
Abstract
While anti-cancer drug treatments are often effective for the clinical management of cancer, these treatments frequently leave behind drug-tolerant persister cancer cells that can ultimately give rise to recurrent disease. Such persistent cancer cells can lie dormant for extended periods of time, going undetected by conventional clinical means. Understanding the mechanisms that such dormant cancer cells use to survive, and the mechanisms that drive emergence from dormancy, is critical to the development of improved therapeutic strategies to prevent and manage disease recurrence. Cancer cells often exhibit metabolic alterations compared to their non-transformed counterparts. An emerging body of evidence supports the notion that dormant cancer cells also have unique metabolic adaptations that may offer therapeutically targetable vulnerabilities. Herein, we review mechanisms through which cancer cells metabolically adapt to persist during drug treatments and develop drug resistance. We also highlight emerging therapeutic strategies to target dormant cancer cells via their metabolic features.

Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Vallette, F. M., Olivier, C., Lézot, F., Oliver, L., Cochonneau, D., Lalier, L., Cartron, P.-F., & Heymann, D. (2019). Dormant, quiescent, tolerant and persister cells: Four synonyms for the same target in cancer. Biochemical Pharmacology, 162, 169–176. https://doi.org/10.1016/j.bcp.2018.11.004
Aguirre-Ghiso, J. A. (2007). Models, mechanisms and clinical evidence for cancer dormancy. Nature Reviews Cancer, 7(11), 834–846. https://doi.org/10.1038/nrc2256
Sosa, M. S., Bragado, P., & Aguirre-Ghiso, J. A. (2014). Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature Reviews Cancer, 14(9), 611–622. https://doi.org/10.1038/nrc3793
Roesch, A., Vultur, A., Bogeski, I., Wang, H., Zimmermann, K. M., Speicher, D., Körbel, C., Laschke, M. W., Gimotty, P. A., Philipp, S. E., et al. (2013). Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell, 23(6), 811–825. https://doi.org/10.1016/j.ccr.2013.05.003
Jin, X., Demere, Z., Nair, K., Ali, A., Ferraro, G. B., Natoli, T., Deik, A., Petronio, L., Tang, A. A., Zhu, C., et al. (2020). A metastasis map of human cancer cell lines. Nature, 588(7837), 331–336. https://doi.org/10.1038/s41586-020-2969-2
Ward, P. S., & Thompson, C. B. (2012). Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell, 21(3), 297–308. https://doi.org/10.1016/j.ccr.2012.02.014
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G., & Thompson, C. B. (2008). The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism, 7(1), 11–20. https://doi.org/10.1016/j.cmet.2007.10.002
DeNicola, G. M., & Cantley, L. C. (2015). Cancer’s fuel choice: New flavors for a picky eater. Molecular cell, 60(4), 514–523. https://doi.org/10.1016/j.molcel.2015.10.018
Reinfeld, B. I., Madden, M. Z., Wolf, M. M., Chytil, A., Bader, J. E., Patterson, A. R., Sugiura, A., Cohen, A. S., Ali, A., Do, B. T., et al. (2021). Cell-programmed nutrient partitioning in the tumour microenvironment. Nature, 593(7858), 282–288. https://doi.org/10.1038/s41586-021-03442-1
Lum, J. J., Bauer, D. E., Kong, M., Harris, M. H., Li, C., Lindsten, T., & Thompson, C. B. (2005). Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell, 120(2), 237–248. https://doi.org/10.1016/j.cell.2004.11.046
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033. https://doi.org/10.1126/science.1160809
Endo, H., Okuyama, H., Ohue, M., & Inoue, M. (2014). Dormancy of cancer cells with suppression of AKT activity contributes to survival in chronic hypoxia. PLOS ONE, 9(6), e98858. https://doi.org/10.1371/journal.pone.0098858
Assaily, W., Rubinger, D. A., Wheaton, K., Lin, Y., Ma, W., Xuan, W., Brown-Endres, L., Tsuchihara, K., Mak, T. W., & Benchimol, S. (2011). ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Molecular Cell, 44(3), 491–501. https://doi.org/10.1016/j.molcel.2011.08.038
Zaugg, K., Yao, Y., Reilly, P. T., Kannan, K., Kiarash, R., Mason, J., Huang, P., Sawyer, S. K., Fuerth, B., Faubert, B., et al. (2011). Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes & Development, 25(10), 1041–1051. https://doi.org/10.1101/gad.1987211
Wang, C., Li, Z., Lu, Y., Du, R., Katiyar, S., Yang, J., Fu, M., Leader, J. E., Quong, A., Novikoff, P. M., et al. (2006). Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proceedings of the National Academy of Sciences, 103(31), 11567–11572. https://doi.org/10.1073/pnas.0603363103
Lopez-Mejia, I. C., Lagarrigue, S., Giralt, A., Martinez-Carreres, L., Zanou, N., Denechaud, P.-D., Castillo-Armengol, J., Chavey, C., Orpinell, M., Delacuisine, B., et al. (2017). CDK4 phosphorylates AMPKα2 to inhibit its activity and repress fatty acid oxidation. Molecular Cell, 68(2), 336–349.e6. https://doi.org/10.1016/j.molcel.2017.09.034
Toyama, E. Q., Herzig, S., Courchet, J., Lewis, T. L., Losón, O. C., Hellberg, K., Young, N. P., Chen, H., Polleux, F., Chan, D. C., et al. (2016). AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science, 351(6270), 275–281. https://doi.org/10.1126/science.aab4138
Camarda, R., Zhou, A. Y., Kohnz, R. A., Balakrishnan, S., Mahieu, C., Anderton, B., Eyob, H., Kajimura, S., Tward, A., Krings, G., et al. (2016). Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nature Medicine, 22(4), 427–432. https://doi.org/10.1038/nm.4055
Madak-Erdogan, Z., Band, S., Zhao, Y. C., Smith, B. P., Kulkoyluoglu-Cotul, E., Zuo, Q., Santaliz Casiano, A., Wrobel, K., Rossi, G., Smith, R. L., et al. (2019). Free fatty acids rewire cancer metabolism in obesity-associated breast cancer via estrogen receptor and mTOR signaling. Cancer Research, canres.2849.2018. https://doi.org/10.1158/0008-5472.CAN-18-2849
Havas, K. M., Milchevskaya, V., Radic, K., Alladin, A., Kafkia, E., Garcia, M., Stolte, J., Klaus, B., Rotmensz, N., Gibson, T. J., et al. (2017). Metabolic shifts in residual breast cancer drive tumor recurrence. Journal of Clinical Investigation, 127(6), 2091–2105. https://doi.org/10.1172/JCI89914
Fox, D. B., Garcia, N. M. G., McKinney, B. J., Lupo, R., Noteware, L. C., Newcomb, R., Liu, J., Locasale, J. W., Hirschey, M. D., & Alvarez, J. V. (2020). NRF2 activation promotes the recurrence of dormant tumour cells through regulation of redox and nucleotide metabolism. Nature Metabolism, 2(4), 318–334. https://doi.org/10.1038/s42255-020-0191-z
Shen, S., Faouzi, S., Souquere, S., Roy, S., Routier, E., Libenciuc, C., André, F., Pierron, G., Scoazec, J.-Y., & Robert, C. (2020). Melanoma persister cells are tolerant to BRAF/MEK inhibitors via ACOX1-mediated fatty acid oxidation. Cell Reports, 33(8), 108421. https://doi.org/10.1016/j.celrep.2020.108421
Hampsch, R. A., Wells, J. D., Traphagen, N. A., McCleery, C. F., Fields, J. L., Shee, K., Dillon, L. M., Pooler, D. B., Lewis, L. D., Demidenko, E., et al. (2020). ampk activation by metformin promotes survival of dormant ER + breast cancer cells. Clinical Cancer Research, 26(14), 3707–3719. https://doi.org/10.1158/1078-0432.CCR-20-0269
Luo, Z., Zang, M., & Guo, W. (2010). AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncology, 6(3), 457–470. https://doi.org/10.2217/fon.09.174
Li, Y., Liang, R., Sun, M., Li, Z., Sheng, H., Wang, J., Xu, P., Liu, S., Yang, W., Lu, B., et al. (2020). AMPK-dependent phosphorylation of HDAC8 triggers PGM1 expression to promote lung cancer cell survival under glucose starvation. Cancer Letters, 478, 82–92. https://doi.org/10.1016/j.canlet.2020.03.007
Naik, P. P., Mukhopadhyay, S., Praharaj, P. P., Bhol, C. S., Panigrahi, D. P., Mahapatra, K. K., Patra, S., Saha, S., Panda, A. K., Panda, K., et al. (2021). Secretory clusterin promotes oral cancer cell survival via inhibiting apoptosis by activation of autophagy in AMPK/mTOR/ULK1 dependent pathway. Life Sciences, 264, 118722. https://doi.org/10.1016/j.lfs.2020.118722
Ferretti, A. C., Tonucci, F. M., Hidalgo, F., Almada, E., Larocca, M. C., & Favre, C. (2016). AMPK and PKA interaction in the regulation of survival of liver cancer cells subjected to glucose starvation. Oncotarget, 7(14), 17815–17828. https://doi.org/10.18632/oncotarget.7404
Chaube, B., Malvi, P., Singh, S. V., Mohammad, N., Viollet, B., & Bhat, M. K. (2015). AMPK maintains energy homeostasis and survival in cancer cells via regulating p38/PGC-1α-mediated mitochondrial biogenesis. Cell Death Discovery, 1(1), 1–11. https://doi.org/10.1038/cddiscovery.2015.63
Peart, T., Valdes, Y. R., Correa, R. J. M., Fazio, E., Bertrand, M., McGee, J., Préfontaine, M., Sugimoto, A., DiMattia, G. E., & Shepherd, T. G. (2015). Intact LKB1 activity is required for survival of dormant ovarian cancer spheroids. Oncotarget, 6(26), 22424–22438.
You, B., Xia, T., Gu, M., Zhang, Z., Zhang, Q., Shen, J., Fan, Y., Yao, H., Pan, S., Lu, Y., et al. (2022). AMPK–mTOR–mediated activation of autophagy promotes formation of dormant polyploid giant cancer cells. Cancer Research, 82(5), 846–858. https://doi.org/10.1158/0008-5472.CAN-21-2342
Lu, Z., Luo, R. Z., Lu, Y., Zhang, X., Yu, Q., Khare, S., Kondo, S., Kondo, Y., Yu, Y., Mills, G. B., et al. (2008). The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. The Journal of Clinical Investigation, 118(12), 3917–3929. https://doi.org/10.1172/JCI35512
Kim, S. M., Nguyen, T. T., Ravi, A., Kubiniok, P., Finicle, B. T., Jayashankar, V., Malacrida, L., Hou, J., Robertson, J., Gao, D., et al. (2018). PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discovery, 8(7), 866–883. https://doi.org/10.1158/2159-8290.CD-17-1215
Glatz, J. F. C., & Luiken, J. J. F. P. (2020). Time for a détente in the war on the mechanism of cellular fatty acid uptake. Journal of Lipid Research, 61(9), 1300–1303. https://doi.org/10.1194/jlr.6192020LTE
Ladanyi, A., Mukherjee, A., Kenny, H. A., Johnson, A., Mitra, A. K., Sundaresan, S., Nieman, K. M., Pascual, G., Benitah, S. A., Montag, A., et al. (2018). Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene, 37(17), 2285–2301. https://doi.org/10.1038/s41388-017-0093-z
Zhao, J., Zhi, Z., Wang, C., Xing, H., Song, G., Yu, X., Zhu, Y., Wang, X., Zhang, X., & Di, Y. (2017). Exogenous lipids promote the growth of breast cancer cells via CD36. Oncology Reports, 38(4), 2105–2115. https://doi.org/10.3892/or.2017.5864
Drury, J., Rychahou, P. G., He, D., Jafari, N., Wang, C., Lee, E. Y., Weiss, H. L., Evers, B. M., & Zaytseva, Y. Y. (2020). Inhibition of fatty acid synthase upregulates expression of cd36 to sustain proliferation of colorectal cancer cells. Frontiers in Oncology, 10 Retrieved from https://www.frontiersin.org/article/10.3389/fonc.2020.01185
Farge, T., Saland, E., de Toni, F., Aroua, N., Hosseini, M., Perry, R., Bosc, C., Sugita, M., Stuani, L., Fraisse, M., et al. (2017). Chemotherapy-resistant human acute myeloid leukemia cells are not enriched for leukemic stem cells but require oxidative metabolism. Cancer Discovery, 7(7), 716–735. https://doi.org/10.1158/2159-8290.CD-16-0441
Pascual, G., Avgustinova, A., Mejetta, S., Martín, M., Castellanos, A., Attolini, C. S.-O., Berenguer, A., Prats, N., Toll, A., Hueto, J. A., et al. (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature, 541(7635), 41–45. https://doi.org/10.1038/nature20791
Landberg, N., von Palffy, S., Askmyr, M., Lilljebjörn, H., Sandén, C., Rissler, M., Mustjoki, S., Hjorth-Hansen, H., Richter, J., Ågerstam, H., et al. (2018). CD36 defines primitive chronic myeloid leukemia cells less responsive to imatinib but vulnerable to antibody-based therapeutic targeting. Haematologica, 103(3), 447–455. https://doi.org/10.3324/haematol.2017.169946
Aloia, A., Müllhaupt, D., Chabbert, C. D., Eberhart, T., Flückiger-Mangual, S., Vukolic, A., Eichhoff, O., Irmisch, A., Alexander, L. T., Scibona, E., et al. (2019). A fatty acid oxidation-dependent metabolic shift regulates the adaptation of BRAF-mutated melanoma to MAPK inhibitors. Clinical Cancer Research, 25(22), 6852–6867. https://doi.org/10.1158/1078-0432.CCR-19-0253
Park, J. H., Vithayathil, S., Kumar, S., Sung, P.-L., Dobrolecki, L. E., Putluri, V., Bhat, V. B., Bhowmik, S. K., Gupta, V., Arora, K., et al. (2016). Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Reports, 14(9), 2154–2165. https://doi.org/10.1016/j.celrep.2016.02.004
Liang, H., & Ward, W. F. (2006). PGC-1alpha: A key regulator of energy metabolism. Advances in Physiology Education, 30(4), 145–151. https://doi.org/10.1152/advan.00052.2006
Ying, H., Kimmelman, A. C., Lyssiotis, C. A., Hua, S., Chu, G. C., Fletcher-Sananikone, E., Locasale, J. W., Son, J., Zhang, H., Coloff, J. L., et al. (2012). Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 149(3), 656–670. https://doi.org/10.1016/j.cell.2012.01.058
Bosc, C., Saland, E., Bousard, A., Gadaud, N., Sabatier, M., Cognet, G., Farge, T., Boet, E., Gotanègre, M., Aroua, N., et al. (2021). Mitochondrial inhibitors circumvent adaptive resistance to venetoclax and cytarabine combination therapy in acute myeloid leukemia. Nature Cancer, 2(11), 1204–1223. https://doi.org/10.1038/s43018-021-00264-y
Henkenius, K., Greene, B. H., Barckhausen, C., Hartmann, R., Märken, M., Kaiser, T., Rehberger, M., Metzelder, S. K., Parak, W. J., Neubauer, A., et al. (2017). Maintenance of cellular respiration indicates drug resistance in acute myeloid leukemia. Leukemia Research, 62, 56–63. https://doi.org/10.1016/j.leukres.2017.09.021
Echeverria, G. V., Ge, Z., Seth, S., Zhang, X., Jeter-Jones, S., Zhou, X., Cai, S., Tu, Y., McCoy, A., Peoples, M., et al. (2019). Resistance to neoadjuvant chemotherapy in triple-negative breast cancer mediated by a reversible drug-tolerant state. Science Translational Medicine, 11(488), 936. https://doi.org/10.1126/scitranslmed.aav0936
Lee, K., Giltnane, J. M., Balko, J. M., Schwarz, L. J., Guerrero-Zotano, A. L., Hutchinson, K. E., Nixon, M. J., Estrada, M. V., Sánchez, V., Sanders, M. E., et al. (2017). MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metabolism, 26(4), 633–647.e7. https://doi.org/10.1016/j.cmet.2017.09.009
Buschhaus, J. M., Humphries, B. A., Eckley, S. S., Robison, T. H., Cutter, A. C., Rajendran, S., Haley, H. R., Bevoor, A. S., Luker, K. E., & Luker, G. D. (2020). Targeting disseminated estrogen-receptor-positive breast cancer cells in bone marrow. Oncogene, 39(34), 5649–5662. https://doi.org/10.1038/s41388-020-01391-z
Franco, J., Balaji, U., Freinkman, E., Witkiewicz, A. K., & Knudsen, E. S. (2016). Metabolic reprogramming of pancreatic cancer mediated by CDK4/6 inhibition elicits unique vulnerabilities. Cell Reports, 14(5), 979–990. https://doi.org/10.1016/j.celrep.2015.12.094
Kunimasa, K., Nagano, T., Shimono, Y., Dokuni, R., Kiriu, T., Tokunaga, S., Tamura, D., Yamamoto, M., Tachihara, M., Kobayashi, K., et al. (2017). Glucose metabolism-targeted therapy and withaferin A are effective for epidermal growth factor receptor tyrosine kinase inhibitor-induced drug-tolerant persisters. Cancer Science, 108(7), 1368–1377. https://doi.org/10.1111/cas.13266
Dörr, J. R., Yu, Y., Milanovic, M., Beuster, G., Zasada, C., Däbritz, J. H. M., Lisec, J., Lenze, D., Gerhardt, A., Schleicher, K., et al. (2013). Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature, 501(7467), 421–425. https://doi.org/10.1038/nature12437
Goldman, A., Khiste, S., Freinkman, E., Dhawan, A., Majumder, B., Mondal, J., Pinkerton, A. B., Eton, E., Medhi, R., Chandrasekar, V., et al. (2019). Targeting tumor phenotypic plasticity and metabolic remodeling in adaptive cross-drug tolerance. Science Signaling, 12(595), eaas8779. https://doi.org/10.1126/scisignal.aas8779
Zhang, W. C., Wells, J. M., Chow, K.-H., Huang, H., Yuan, M., Saxena, T., Melnick, M. A., Politi, K., Asara, J. M., Costa, D. B., et al. (2019). miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma. Nature Metabolism, 1(4), 460–474. https://doi.org/10.1038/s42255-019-0052-9
Patel, S. B., Nemkov, T., Stefanoni, D., Benavides, G. A., Bassal, M. A., Crown, B. L., Matkins, V. R., Camacho, V., Kuznetsova, V., Hoang, A. T., et al. (2021). Metabolic alterations mediated by STAT3 promotes drug persistence in CML. Leukemia, 35(12), 3371–3382. https://doi.org/10.1038/s41375-021-01315-0
Paredes, F., Williams, H. C., & San Martin, A. (2021). Metabolic adaptation in hypoxia and cancer. Cancer Letters, 502, 133–142. https://doi.org/10.1016/j.canlet.2020.12.020
Harper, M.-E., Antoniou, A., Villalobos-Menuey, E., Russo, A., Trauger, R., Vendemelio, M., George, A., Bartholomew, R., Carlo, D., Shaikh, A., et al. (2002). Characterization of a novel metabolic strategy used by drug-resistant tumor cells. The FASEB Journal, 16(12), 1550–1557. https://doi.org/10.1096/fj.02-0541com
Oren, Y., Tsabar, M., Cuoco, M. S., Amir-Zilberstein, L., Cabanos, H. F., Hütter, J.-C., Hu, B., Thakore, P. I., Tabaka, M., Fulco, C. P., et al. (2021). Cycling cancer persister cells arise from lineages with distinct programs. Nature, 1–7. https://doi.org/10.1038/s41586-021-03796-6
Pike, L. S., Smift, A. L., Croteau, N. J., Ferrick, D. A., & Wu, M. (2011). Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochimica et Biophysica Acta (BBA). Bioenergetics, 1807(6), 726–734. https://doi.org/10.1016/j.bbabio.2010.10.022
Jeon, S.-M., Chandel, N. S., & Hay, N. (2012). AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature, 485(7400), 661–665. https://doi.org/10.1038/nature11066
Kaplan, O., Navon, G., Lyon, R. C., Faustino, P. J., Straka, E. J., & Cohen, J. S. (1990). Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: Toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Research, 50(3), 544–551.
La Belle Flynn, A., Calhoun, B. C., Sharma, A., Chang, J. C., Almasan, A., & Schiemann, W. P. (2019). Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nature Communications, 10(1), 3668. https://doi.org/10.1038/s41467-019-11640-9
Morris, V. L., Tuck, A. B., Wilson, S. M., Percy, D., & Chambers, A. F. (1993). Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clinical & Experimental Metastasis, 11(1), 103–112. https://doi.org/10.1007/BF00880071
Rusu, P., Shao, C., Neuerburg, A., Acikgöz, A. A., Wu, Y., Zou, P., Phapale, P., Shankar, T. S., Döring, K., Dettling, S., et al. (2019). GPD1 specifically marks dormant glioma stem cells with a distinct metabolic profile. Cell Stem Cell, 25(2), 241–257.e8. https://doi.org/10.1016/j.stem.2019.06.004
Chino, H. (1957). Conversion of glycogen to sorbitol and glycerol in the diapause egg of the bombyx silkworm. Nature, 180(4586), 606–607. https://doi.org/10.1038/180606b0
Vyas, S., Zaganjor, E., & Haigis, M. C. (2016). Mitochondria and cancer. Cell, 166(3), 555–566. https://doi.org/10.1016/j.cell.2016.07.002
Viale, A., Pettazzoni, P., Lyssiotis, C. A., Ying, H., Sánchez, N., Marchesini, M., Carugo, A., Green, T., Seth, S., Giuliani, V., et al. (2014). Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature, 514(7524), 628–632. https://doi.org/10.1038/nature13611
Samuels, A. L., Heng, J. Y., Beesley, A. H., & Kees, U. R. (2014). Bioenergetic modulation overcomes glucocorticoid resistance in T-lineage acute lymphoblastic leukaemia. British Journal of Haematology, 165(1), 57–66. https://doi.org/10.1111/bjh.12727
Molina, J. R., Sun, Y., Protopopova, M., Gera, S., Bandi, M., Bristow, C., McAfoos, T., Morlacchi, P., Ackroyd, J., Agip, A. N. A., et al. (2018). An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nature Medicine, 24(7), 1036–1046. https://doi.org/10.1038/s41591-018-0052-4
Vyas, A., Harbison, R. A., Faden, D. L., Kubik, M., Palmer, D., Zhang, Q., Osmanbeyoglu, H. U., Kiselyov, K., Méndez, E., & Duvvuri, U. (2021). Recurrent human papillomavirus–related head and neck cancer undergoes metabolic reprogramming and is driven by oxidative phosphorylation. Clinical Cancer Research, 27(22), 6250–6264. https://doi.org/10.1158/1078-0432.CCR-20-4789
Yap, T. A., Rodon Ahnert, J., Piha-Paul, S. A., Fu, S., Janku, F., Karp, D. D., Naing, A., Ileana Dumbrava, E. E., Pant, S., Subbiah, V., et al. (2019). Phase I trial of IACS-010759 (IACS), a potent, selective inhibitor of complex I of the mitochondrial electron transport chain, in patients (pts) with advanced solid tumors. Journal of Clinical Oncology, 37(15_suppl), 3014–3014. https://doi.org/10.1200/JCO.2019.37.15_suppl.3014
Nouri, K., Feng, Y., & Schimmer, A. D. (2020). Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death & Disease, 11(10), 1–12. https://doi.org/10.1038/s41419-020-03062-z
Graves, P. R., Aponte-Collazo, L. J., Fennell, E. M. J., Graves, A. C., Hale, A. E., Dicheva, N., Herring, L. E., Gilbert, T. S. K., East, M. P., McDonald, I. M., et al. (2019). Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chemical Biology, 14(5), 1020–1029. https://doi.org/10.1021/acschembio.9b00222
Shi, Y., Lim, S. K., Liang, Q., Iyer, S. V., Wang, H.-Y., Wang, Z., Xie, X., Sun, D., Chen, Y.-J., Tabar, V., et al. (2019). Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature, 567(7748), 341–346. https://doi.org/10.1038/s41586-019-0993-x
Zhang, X., Fryknäs, M., Hernlund, E., Fayad, W., De Milito, A., Olofsson, M. H., Gogvadze, V., Dang, L., Påhlman, S., Schughart, L. A. K., et al. (2014). Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nature Communications, 5(1), 1–14. https://doi.org/10.1038/ncomms4295
Sharon, D., Cathelin, S., Mirali, S., Di Trani, J. M., Yanofsky, D. J., Keon, K. A., Rubinstein, J. L., Schimmer, A. D., Ketela, T., & Chan, S. M. (2019). Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Science Translational Medicine, 11(516), 2863. https://doi.org/10.1126/scitranslmed.aax2863
Kuntz, E. M., Baquero, P., Michie, A. M., Dunn, K., Tardito, S., Holyoake, T. L., Helgason, G. V., & Gottlieb, E. (2017). Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nature Medicine, 23(10), 1234–1240. https://doi.org/10.1038/nm.4399
Taanman, J.-W. (1999). The mitochondrial genome: structure, transcription, translation and replication. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1410(2), 103–123. https://doi.org/10.1016/S0005-2728(98)00161-3
Bonekamp, N. A., Peter, B., Hillen, H. S., Felser, A., Bergbrede, T., Choidas, A., Horn, M., Unger, A., Di Lucrezia, R., Atanassov, I., et al. (2020). Small-molecule inhibitors of human mitochondrial DNA transcription. Nature, 588(7839), 712–716. https://doi.org/10.1038/s41586-020-03048-z
Samudio, I., Harmancey, R., Fiegl, M., Kantarjian, H., Konopleva, M., Korchin, B., Kaluarachchi, K., Bornmann, W., Duvvuri, S., Taegtmeyer, H., et al. (2010). Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. The Journal of Clinical Investigation, 120(1), 142–156. https://doi.org/10.1172/JCI38942
Caro, P., Kishan, A. U., Norberg, E., Stanley, I., Chapuy, B., Ficarro, S. B., Polak, K., Tondera, D., Gounarides, J., Yin, H., et al. (2012). Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B-cell lymphoma. Cancer cell, 22(4), 547–560. https://doi.org/10.1016/j.ccr.2012.08.014
Menendez, J. A., & Lupu, R. (2017). Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opinion on Therapeutic Targets, 21(11), 1001–1016. https://doi.org/10.1080/14728222.2017.1381087
Heuer, T. S., Ventura, R., Mordec, K., Lai, J., Fridlib, M., Buckley, D., & Kemble, G. (2017). FASN inhibition and taxane treatment combine to enhance anti-tumor efficacy in diverse xenograft tumor models through disruption of tubulin palmitoylation and microtubule organization and fasn inhibition-mediated effects on oncogenic signaling and gene expression. EBioMedicine, 16, 51–62. https://doi.org/10.1016/j.ebiom.2016.12.012
Zaytseva, Y. Y., Rychahou, P. G., Le, A.-T., Scott, T. L., Flight, R. M., Kim, J. T., Harris, J., Liu, J., Wang, C., Morris, A. J., et al. (2018). Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget, 9(37), 24787–24800. https://doi.org/10.18632/oncotarget.25361
Falchook, G., Infante, J., Arkenau, H.-T., Patel, M. R., Dean, E., Borazanci, E., Brenner, A., Cook, N., Lopez, J., Pant, S., et al. (2021). First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. eClinicalMedicine, 34. https://doi.org/10.1016/j.eclinm.2021.100797
Feng, W. W., Wilkins, O., Bang, S., Ung, M., Li, J., An, J., del Genio, C., Canfield, K., DiRenzo, J., Wells, W., et al. (2019). CD36-mediated metabolic rewiring of breast cancer cells promotes resistance to HER2-targeted therapies. Cell Reports, 29(11), 3405–3420.e5. https://doi.org/10.1016/j.celrep.2019.11.008
Hernlund, E., Ihrlund, L. S., Khan, O., Ates, Y. O., Linder, S., Panaretakis, T., & Shoshan, M. C. (2008). Potentiation of chemotherapeutic drugs by energy metabolism inhibitors 2-deoxyglucose and etomoxir. International Journal of Cancer, 123(2), 476–483. https://doi.org/10.1002/ijc.23525
Holubarsch, C. J. F., Rohrbach, M., Karrasch, M., Boehm, E., Polonski, L., Ponikowski, P., & Rhein, S. (2007). A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clinical Science, 113(4), 205–212. https://doi.org/10.1042/CS20060307
Zachar, Z., Marecek, J., Maturo, C., Gupta, S., Stuart, S. D., Howell, K., Schauble, A., Lem, J., Piramzadian, A., Karnik, S., et al. (2011). Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. Journal of Molecular Medicine, 89(11), 1137. https://doi.org/10.1007/s00109-011-0785-8
Arnold, C., Demuth, P., Seiwert, N., Wittmann, S., Boengler, K., Rasenberger, B., Christmann, M., Huber, M., Brunner, T., Linnebacher, M., et al. (2022). The mitochondrial disruptor devimistat (CPI-613) synergizes with genotoxic anticancer drugs in colorectal cancer therapy in a Bim-dependent manner. Molecular Cancer Therapeutics, 21(1), 100–112. https://doi.org/10.1158/1535-7163.MCT-21-0393
Pardee, T. S., Luther, S., Buyse, M., Powell, B. L., & Cortes, J. (2019). Devimistat in combination with high dose cytarabine and mitoxantrone compared with high dose cytarabine and mitoxantrone in older patients with relapsed/refractory acute myeloid leukemia: ARMADA 2000 Phase III study. Future Oncology, 15(28), 3197–3208. https://doi.org/10.2217/fon-2019-0201
Anderson, R., Miller, L. D., Isom, S., Chou, J. W., Pladna, K. M., Schramm, N. J., Ellis, L. R., Howard, D. S., Bhave, R. R., Manuel, M., et al. (2022). Phase II trial of cytarabine and mitoxantrone with devimistat in acute myeloid leukemia. Nature Communications, 13(1), 1673. https://doi.org/10.1038/s41467-022-29039-4
Tsvetkov, P., Coy, S., Petrova, B., Dreishpoon, M., Verma, A., Abdusamad, M., Rossen, J., Joesch-Cohen, L., Humeidi, R., Spangler, R. D., et al. (2022). Copper induces cell death by targeting lipoylated TCA cycle proteins. Science, 375(6586), 1254–1261. https://doi.org/10.1126/science.abf0529
Tsvetkov, P., Detappe, A., Cai, K., Keys, H. R., Brune, Z., Ying, W., Thiru, P., Reidy, M., Kugener, G., Rossen, J., et al. (2019). Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nature Chemical Biology, 15(7), 681–689. https://doi.org/10.1038/s41589-019-0291-9
de Groot, S., Pijl, H., van der Hoeven, J. J. M., & Kroep, J. R. (2019). Effects of short-term fasting on cancer treatment. Journal of Experimental & Clinical Cancer Research : CR, 38, 209. https://doi.org/10.1186/s13046-019-1189-9
Caffa, I., Spagnolo, V., Vernieri, C., Valdemarin, F., Becherini, P., Wei, M., Brandhorst, S., Zucal, C., Driehuis, E., Ferrando, L., et al. (2020). Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature, 583(7817), 620–624. https://doi.org/10.1038/s41586-020-2502-7
Thandapani, P., Kloetgen, A., Witkowski, M. T., Glytsou, C., Lee, A. K., Wang, E., Wang, J., LeBoeuf, S. E., Avrampou, K., Papagiannakopoulos, T., et al. (2022). Valine tRNA levels and availability regulate complex I assembly in leukaemia. Nature, 601(7893), 428–433. https://doi.org/10.1038/s41586-021-04244-1
Rose, D. P. (1997). Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. The American Journal of Clinical Nutrition, 66(6), 1513S–1522S. https://doi.org/10.1093/ajcn/66.6.1513S
Donnelly, C., Olsen, A. M., Lewis, L. D., Eisenberg, B. L., Eastman, A., & Kinlaw, W. B. (2009). Conjugated Linoleic Acid (CLA) inhibits expression of the spot 14 (THRSP) and fatty acid synthase genes and impairs the growth of human breast cancer and liposarcoma cells. Nutrition and Cancer, 61(1), 114–122. https://doi.org/10.1080/01635580802348666
McGowan, M. M., Eisenberg, B. L., Lewis, L. D., Froehlich, H. M., Wells, W. A., Eastman, A., Kuemmerle, N. B., Rosenkrantz, K. M., Barth, R. J., Schwartz, G. N., et al. (2013). A proof of principle clinical trial to determine whether conjugated linoleic acid modulates the lipogenic pathway in human breast cancer tissue. Breast Cancer Research and Treatment, 138(1), 175–183. https://doi.org/10.1007/s10549-013-2446-9
Mayer, E. L., Dueck, A. C., Martin, M., Rubovszky, G., Burstein, H. J., Bellet-Ezquerra, M., Miller, K. D., Zdenkowski, N., Winer, E. P., Pfeiler, G., et al. (2021). Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. The Lancet Oncology, 22(2), 212–222. https://doi.org/10.1016/S1470-2045(20)30642-2
Vansteenkiste, J. F., Cho, B. C., Vanakesa, T., De Pas, T., Zielinski, M., Kim, M. S., Jassem, J., Yoshimura, M., Dahabreh, J., Nakayama, H., et al. (2016). Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. Oncology, 17(6), 822–835. https://doi.org/10.1016/S1470-2045(16)00099-1
Alberts, S. R., Sargent, D. J., Nair, S., Mahoney, M. R., Mooney, M., Thibodeau, S. N., Smyrk, T. C., Sinicrope, F. A., Chan, E., Gill, S., et al. (2012). Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA, 307(13), 1383–1393. https://doi.org/10.1001/jama.2012.385
Nielsen, T. O., Jensen, M.-B., Burugu, S., Gao, D., Jørgensen, C. L. T., Balslev, E., & Ejlertsen, B. (2017). High-risk premenopausal luminal a breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: Results from the DBCG77B clinical trial. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 23(4), 946–953. https://doi.org/10.1158/1078-0432.CCR-16-1278
Haas, N. B., Manola, J., Uzzo, R. G., Flaherty, K. T., Wood, C. G., Kane, C., Jewett, M., Dutcher, J. P., Atkins, M. B., Pins, M., et al. (2016). Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet (London, England), 387(10032), 2008–2016. https://doi.org/10.1016/S0140-6736(16)00559-6
de Gramont, A., Van Cutsem, E., Schmoll, H.-J., Tabernero, J., Clarke, S., Moore, M. J., Cunningham, D., Cartwright, T. H., Hecht, J. R., Rivera, F., et al. (2012). Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase 3 randomised controlled trial. The Lancet Oncology, 13(12), 1225–1233. https://doi.org/10.1016/S1470-2045(12)70509-0
Allegra, C. J., Yothers, G., O’Connell, M. J., Sharif, S., Petrelli, N. J., Colangelo, L. H., Atkins, J. N., Seay, T. E., Fehrenbacher, L., Goldberg, R. M., et al. (2011). Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 29(1), 11–16. https://doi.org/10.1200/JCO.2010.30.0855
Acknowledgements
This work was supported by the National Cancer Institute (R01CA211869, R01CA200994, R01CA262232, R01CA267691 to TWM; Dartmouth Cancer Center Support Grant P30CA023108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tau, S., Miller, T.W. The role of cancer cell bioenergetics in dormancy and drug resistance. Cancer Metastasis Rev 42, 87–98 (2023). https://doi.org/10.1007/s10555-023-10081-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-023-10081-7