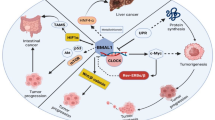
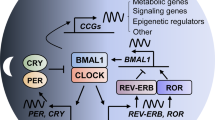
Abstract
The circadian clock is a timekeeping system for numerous biological rhythms that contribute to the regulation of numerous homeostatic processes in humans. Disruption of circadian rhythms influences physiology and behavior and is associated with adverse health outcomes, especially cancer. However, the underlying molecular mechanisms of circadian disruption-associated cancer initiation and development remain unclear. It is essential to construct good circadian disruption models to uncover and validate the detailed molecular clock framework of circadian disruption in cancer development and progression. Mouse models are the most widely used in circadian studies due to their relatively small size, fast reproduction cycle, easy genome manipulation, and economic practicality. Here, we reviewed the current mouse models of circadian disruption, including suprachiasmatic nuclei destruction, genetic engineering, light disruption, sleep deprivation, and other lifestyle factors in our understanding of the crosstalk between circadian rhythms and oncogenic signaling, as well as the molecular mechanisms of circadian disruption that promotes cancer growth. We focused on the discoveries made with the nocturnal mouse, diurnal human being, and cell culture and provided several circadian rhythm-based cancer therapeutic strategies.







Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- SCN:
-
Suprachiasmatic nuclei
- TTFLs:
-
Transcription-translation negative feedback loops
- ARNTL:
-
Hydrocarbon receptor nuclear translocator-like protein 1
- BMAL1:
-
Brain and muscle ARNT-like protein1
- CLOCK:
-
Circadian locomotor output cycles kaput
- PER1:
-
Period 1
- PER2:
-
Period 2
- PER3:
-
Period 3
- CRY1:
-
Cryptochrome 1
- CRY2:
-
Cryptochrome 2
- NR1D1:
-
Nuclear receptor subfamily 1 group D member 1
- NR1D2:
-
Nuclear receptor subfamily 1 group D member 2
- CK1δ:
-
Casein kinase 1δ
- ROR:
-
Retinoic acid receptor-related orphan receptor
- RORE:
-
ROR-response element
- PAR-bZIP:
-
Proline and acidic amino acid-rich basic leucine zipper
- DBP:
-
D-element-binding protein
- TEF:
-
Thyrotrophic embryonic factor
- HLF:
-
Hepatic leukemia factor
- NFIL3:
-
Nuclear factor interleukin 3-regulated
- AMPK:
-
AMP-activated protein kinase
- mTOR:
-
Mechanistic target of rapamycin
- HIF1α:
-
Hypoxia-inducible factor-1α
- MAPKs:
-
Mitogen-activated protein kinases
- MAPK8:
-
MAP kinases 8
- GSK3β:
-
Glycogen synthase kinase 3 beta
- ATF4:
-
Activating transcription factor 4
- CRE:
-
CAMP-response element
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- PPARs:
-
Peroxisome proliferator-activated receptors
- RXR:
-
Retinoid X receptor
- PPRE:
-
PPAR response element
- PGC-1α:
-
PPARγ coactivator 1α
- CDK1:
-
Cyclin-dependent kinase 1
- CREB:
-
CAMP response element-binding protein
- CBP:
-
CREB-binding protein
- HDAC3:
-
Histone deacetylase 3
- ipRGCs:
-
intrinsically photosensitive retinal ganglion cells
- IGL:
-
Intergeniculate leaflet
- GOS:
-
Glasgow osteosarcoma
- AAV:
-
Adeno-associated virus
- KD:
-
Knockdown
- KO:
-
Knockout
- Cre:
-
cyclization recombination recombinase
- FRT:
-
Flippase recognition target
- ABC:
-
ATP-binding cassette
- ABCG5:
-
ABC subfamily G member 5
- ABCG8:
-
ABC subfamily G member 8
- NAFLD:
-
Non-alcoholic fatty liver disease
- CRC:
-
Colorectal cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- HCC:
-
Hepatocellular carcinoma
- CAR:
-
Constitutive androstane receptor
- ROS:
-
Reactive oxygen species
- LSC:
-
Leukemia stem cells
- EMT:
-
Epithelial to mesenchymal transition
- OSCC:
-
Oral squamous cell carcinoma
- GSC:
-
Glioma stem-like cells
- Aldh3a1:
-
Aldehyde dehydrogenase 3a1
- DKO:
-
Double KO
- DEN:
-
Diethylnitrosamine
- RHT:
-
Retinohypothalamic tract
- CJL:
-
Chronic jet lag
- LD:
-
Light-dark
- DD:
-
Constant darkness
- NK:
-
Nature killer
- LL:
-
Constant light
- LAN:
-
Light at night
- DOX:
-
Doxorubicin
- BMI:
-
Body mass index
- WTE:
-
Wrong time eating
- Th17:
-
T-helper cell 17
- Treg:
-
Regulatory T
- TME:
-
tumor microenvironment
- TRE:
-
Time-restricted eating
- HFD:
-
High-fat diet
- RTE:
-
Right time eating
- SD:
-
Sleep deprivation
- DCs:
-
dendritic cells
- MPM:
-
Multiple platform method
- MDSCs:
-
myeloid-derived suppressor cells
- PSD:
-
Paradoxical sleep deprivation
- IL-1β:
-
interleukin-1β
- ASD:
-
Acute sleep deprivation
- TAMs:
-
tumor-associated macrophages
- CSD:
-
Chronic sleep deprivation
- SF:
-
Sleep fragmentation
- NAT:
-
N-acetyltransferase
- HIOMT:
-
Hydroxy indole-O-methyltransferase
- UEGs:
-
Universally expressed genes
- GABA:
-
G-Aminobutyric acid
- PACAP:
-
Cyclase-activating peptide
- ANS:
-
Autonomic nervous system
- PVN-SCG:
-
Paraventricular nucleus-superior cervical ganglia
- TTX:
-
Tetrodotoxin
- VIP:
-
Vasoactive intestinal peptide
- CNO:
-
clozapine N-oxide
- DREADD:
-
Designer receptors exclusively activated by designer drugs
- Tc17 CD8+ :
-
type 17 effector
- NOB:
-
Natural flavonoid nobiletin
- PFKFB3:
-
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
- DIO:
-
Diet-induced obese
- G6PD:
-
glucose-6-phosphate 1-dehydrogenase
References
Shafi, A. A., & Knudsen, K. E. (2019). Cancer and the circadian clock. Cancer Research, 79(15), 3806–3814. https://doi.org/10.1158/0008-5472.Can-19-0566
Huang, W., Ramsey, K. M., Marcheva, B., & Bass, J. (2011). Circadian rhythms, sleep, and metabolism. The Journal of Clinical Investigation, 121(6), 2133–2141. https://doi.org/10.1172/jci46043
Panda, S. (2016). Circadian physiology of metabolism. Science, 354(6315), 1008–1015. https://doi.org/10.1126/science.aah4967
Firsov, D., & Bonny, O. (2018). Circadian rhythms and the kidney. Nature Reviews. Nephrology, 14(10), 626–635. https://doi.org/10.1038/s41581-018-0048-9
Brancaccio, M., Edwards, M. D., Patton, A. P., Smyllie, N. J., Chesham, J. E., Maywood, E. S., et al. (2019). Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science, 363(6423), 187–192. https://doi.org/10.1126/science.aat4104
Millar, A. J. (2016). The intracellular dynamics of circadian clocks reach for the light of ecology and evolution. Annual Review of Plant Biology, 67, 595–618. https://doi.org/10.1146/annurev-arplant-043014-115619
Poggiogalle, E., Jamshed, H., & Peterson, C. M. (2018). Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism, 84, 11–27. https://doi.org/10.1016/j.metabol.2017.11.017
Ruan, G. X., Gamble, K. L., Risner, M. L., Young, L. A., & McMahon, D. G. (2012). Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators. PLoS ONE, 7(6), e38985. https://doi.org/10.1371/journal.pone.0038985
St John, P. C., Hirota, T., Kay, S. A., & Doyle, F. J., 3rd. (2014). Spatiotemporal separation of PER and CRY posttranslational regulation in the mammalian circadian clock. Proc Natl Acad Sci USA, 111(5), 2040–2045. https://doi.org/10.1073/pnas.1323618111
Challet, E. (2019). The circadian regulation of food intake. Nature Reviews. Endocrinology, 15(7), 393–405. https://doi.org/10.1038/s41574-019-0210-x
Lee, J., Lee, S., Chung, S., Park, N., Son, G. H., An, H., et al. (2016). Identification of a novel circadian clock modulator controlling BMAL1 expression through a ROR/REV-ERB-response element-dependent mechanism. Biochemical and Biophysical Research Communications, 469(3), 580–586. https://doi.org/10.1016/j.bbrc.2015.12.030
Gerhart-Hines, Z., & Lazar, M. A. (2015). Rev-erbα and the circadian transcriptional regulation of metabolism. Diabetes Obes Metab, 17 Suppl 1(0 1), 12–16. https://doi.org/10.1111/dom.12510
Kojetin, D. J., & Burris, T. P. (2014). REV-ERB and ROR nuclear receptors as drug targets. Nature Reviews. Drug Discovery, 13(3), 197–216. https://doi.org/10.1038/nrd4100
Mohawk, J. A., Green, C. B., & Takahashi, J. S. (2012). Central and peripheral circadian clocks in mammals. Annual Review of Neuroscience, 35, 445–462. https://doi.org/10.1146/annurev-neuro-060909-153128
Zhou, L., Zhang, Z., Nice, E., Huang, C., Zhang, W., & Tang, Y. (2022). Circadian rhythms and cancers: The intrinsic links and therapeutic potentials. Journal of Hematology & Oncology, 15(1), 21. https://doi.org/10.1186/s13045-022-01238-y
Lamia, K. A., Sachdeva, U. M., DiTacchio, L., Williams, E. C., Alvarez, J. G., Egan, D. F., et al. (2009). AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science, 326(5951), 437–440. https://doi.org/10.1126/science.1172156
Um, J. H., Yang, S., Yamazaki, S., Kang, H., Viollet, B., Foretz, M., et al. (2007). Activation of 5’-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. Journal of Biological Chemistry, 282(29), 20794–20798. https://doi.org/10.1074/jbc.C700070200
Ramanathan, C., Kathale, N. D., Liu, D., Lee, C., Freeman, D. A., Hogenesch, J. B., et al. (2018). mTOR signaling regulates central and peripheral circadian clock function. PLoS Genetics, 14(5), e1007369. https://doi.org/10.1371/journal.pgen.1007369
Lipton, J. O., Boyle, L. M., Yuan, E. D., Hochstrasser, K. J., Chifamba, F. F., Nathan, A., et al. (2017). Aberrant proteostasis of BMAL1 underlies circadian abnormalities in a paradigmatic mTOR-opathy. Cell Reports, 20(4), 868–880. https://doi.org/10.1016/j.celrep.2017.07.008
Peek, C. B., Levine, D. C., Cedernaes, J., Taguchi, A., Kobayashi, Y., Tsai, S. J., et al. (2017). Circadian clock interaction with HIF1alpha mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metabolism, 25(1), 86–92. https://doi.org/10.1016/j.cmet.2016.09.010
Magnelli, L., Schiavone, N., Staderini, F., Biagioni, A., & Papucci, L. (2020). MAP kinases pathways in gastric cancer. Int J Mol Sci, 21(8). https://doi.org/10.3390/ijms21082893
Yoshitane, H., Honma, S., Imamura, K., Nakajima, H., Nishide, S. Y., Ono, D., et al. (2012). JNK regulates the photic response of the mammalian circadian clock. EMBO Reports, 13(5), 455–461. https://doi.org/10.1038/embor.2012.37
Sahar, S., Zocchi, L., Kinoshita, C., Borrelli, E., & Sassone-Corsi, P. (2010). Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS ONE, 5(1), e8561. https://doi.org/10.1371/journal.pone.0008561
Jiang, W., Zhao, S., Jiang, X., Zhang, E., Hu, G., Hu, B., et al. (2016). The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Letters, 371(2), 314–325. https://doi.org/10.1016/j.canlet.2015.12.002
Miki, T., Matsumoto, T., Zhao, Z., & Lee, C. C. (2013). p53 regulates Period2 expression and the circadian clock. Nature Communications, 4, 2444. https://doi.org/10.1038/ncomms3444
Koyanagi, S., Hamdan, A. M., Horiguchi, M., Kusunose, N., Okamoto, A., Matsunaga, N., et al. (2011). cAMP-response element (CRE)-mediated transcription by activating transcription factor-4 (ATF4) is essential for circadian expression of the Period2 gene. Journal of Biological Chemistry, 286(37), 32416–32423. https://doi.org/10.1074/jbc.M111.258970
He, F., Antonucci, L., & Karin, M. (2020). NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis, 41(4), 405–416. https://doi.org/10.1093/carcin/bgaa039
He, F., Antonucci, L., Yamachika, S., Zhang, Z., Taniguchi, K., Umemura, A., et al. (2020). NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly. Journal of Hepatology, 72(6), 1182–1195. https://doi.org/10.1016/j.jhep.2020.01.023
Early, J. O., Menon, D., Wyse, C. A., Cervantes-Silva, M. P., Zaslona, Z., Carroll, R. G., et al. (2018). Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci U S A, 115(36), E8460–E8468. https://doi.org/10.1073/pnas.1800431115
Wible, R. S., Ramanathan, C., Sutter, C. H., Olesen, K. M., Kensler, T. W., Liu, A. C., et al. (2018). NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife, 7. https://doi.org/10.7554/eLife.31656
Chen, L., & Yang, G. (2014). PPARs integrate the mammalian clock and energy metabolism. PPAR Research, 2014, 653017. https://doi.org/10.1155/2014/653017
McNamara, P., Seo, S. B., Rudic, R. D., Sehgal, A., Chakravarti, D., & FitzGerald, G. A. (2001). Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: A humoral mechanism to reset a peripheral clock. Cell, 105(7), 877–889. https://doi.org/10.1016/s0092-8674(01)00401-9
Oishi, K., Shirai, H., & Ishida, N. (2005). CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. The Biochemical Journal, 386(Pt 3), 575–581. https://doi.org/10.1042/bj20041150
Schmutz, I., Ripperger, J. A., Baeriswyl-Aebischer, S., & Albrecht, U. (2010). The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes & Development, 24(4), 345–357. https://doi.org/10.1101/gad.564110
Wang, S., Lin, Y., Gao, L., Yang, Z., Lin, J., Ren, S., et al. (2022). PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics, 12(4), 1589–1606. https://doi.org/10.7150/thno.69054
Li, S., & Lin, J. D. (2015). Transcriptional control of circadian metabolic rhythms in the liver. Diabetes Obes Metab, 17 Suppl 1(0 1), 33–38. https://doi.org/10.1111/dom.12520
Zhao, X., Hirota, T., Han, X., Cho, H., Chong, L. W., Lamia, K., et al. (2016). Circadian amplitude regulation via FBXW7-targeted REV-ERBα degradation. Cell, 165(7), 1644–1657. https://doi.org/10.1016/j.cell.2016.05.012
Kwak, Y., Jeong, J., Lee, S., Park, Y. U., Lee, S. A., Han, D. H., et al. (2013). Cyclin-dependent kinase 5 (Cdk5) regulates the function of CLOCK protein by direct phosphorylation. Journal of Biological Chemistry, 288(52), 36878–36889. https://doi.org/10.1074/jbc.M113.494856
Ou, J., Li, H., Qiu, P., Li, Q., Chang, H. C., & Tang, Y. C. (2019). CDK9 modulates circadian clock by attenuating REV-ERBα activity. Biochemical and Biophysical Research Communications, 513(4), 967–973. https://doi.org/10.1016/j.bbrc.2019.04.043
Lee, Y., Lee, J., Kwon, I., Nakajima, Y., Ohmiya, Y., Son, G. H., et al. (2010). Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. Journal of Cell Science, 123(Pt 20), 3547–3557. https://doi.org/10.1242/jcs.070300
Shi, G., Xie, P., Qu, Z., Zhang, Z., Dong, Z., An, Y., et al. (2016). Distinct roles of HDAC3 in the core circadian negative feedback loop are critical for clock function. Cell Reports, 14(4), 823–834. https://doi.org/10.1016/j.celrep.2015.12.076
Travis, R. C., Balkwill, A., Fensom, G. K., Appleby, P. N., Reeves, G. K., Wang, X. S., et al. (2016). Night shift work and breast cancer incidence: Three prospective studies and meta-analysis of published studies. J Natl Cancer Inst, 108(12). https://doi.org/10.1093/jnci/djw169
Lin, X., Chen, W., Wei, F., Ying, M., Wei, W., & Xie, X. (2015). Night-shift work increases morbidity of breast cancer and all-cause mortality: A meta-analysis of 16 prospective cohort studies. Sleep Medicine, 16(11), 1381–1387. https://doi.org/10.1016/j.sleep.2015.02.543
Cordina-Duverger, E., Menegaux, F., Popa, A., Rabstein, S., Harth, V., Pesch, B., et al. (2018). Night shift work and breast cancer: A pooled analysis of population-based case-control studies with complete work history. European Journal of Epidemiology, 33(4), 369–379. https://doi.org/10.1007/s10654-018-0368-x
Mancio, J., Leal, C., Ferreira, M., Norton, P., & Lunet, N. (2018). Does the association of prostate cancer with night-shift work differ according to rotating vs. fixed schedule? A systematic review and meta-analysis. Prostate Cancer Prostatic Dis, 21(3), 337–344. https://doi.org/10.1038/s41391-018-0040-2
Hansen, J. (2017). Night shift work and risk of breast cancer. Curr Environ Health Rep, 4(3), 325–339. https://doi.org/10.1007/s40572-017-0155-y
Arriaga, J. M., & Abate-Shen, C. (2019). Genetically engineered mouse models of prostate cancer in the postgenomic era. Cold Spring Harb Perspect Med, 9(2). https://doi.org/10.1101/cshperspect.a030528
Walrath, J. C., Hawes, J. J., Van Dyke, T., & Reilly, K. M. (2010). Genetically engineered mouse models in cancer research. Advances in Cancer Research, 106, 113–164. https://doi.org/10.1016/s0065-230x(10)06004-5
Potter, G. D., Skene, D. J., Arendt, J., Cade, J. E., Grant, P. J., & Hardie, L. J. (2016). Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocrine Reviews, 37(6), 584–608. https://doi.org/10.1210/er.2016-1083
Hastings, M. H., Maywood, E. S., & Brancaccio, M. (2018). Generation of circadian rhythms in the suprachiasmatic nucleus. Nature Reviews Neuroscience, 19(8), 453–469. https://doi.org/10.1038/s41583-018-0026-z
Welsh, D. K., Takahashi, J. S., & Kay, S. A. (2010). Suprachiasmatic nucleus: Cell autonomy and network properties. Annual Review of Physiology, 72, 551–577. https://doi.org/10.1146/annurev-physiol-021909-135919
Herzog, E. D. (2007). Neurons and networks in daily rhythms. Nature Reviews Neuroscience, 8(10), 790–802. https://doi.org/10.1038/nrn2215
Golombek, D. A., & Rosenstein, R. E. (2010). Physiology of circadian entrainment. Physiological Reviews, 90(3), 1063–1102. https://doi.org/10.1152/physrev.00009.2009
Riedel, C. S., Georg, B., Fahrenkrug, J., & Hannibal, J. (2020). Altered light induced EGR1 expression in the SCN of PACAP deficient mice. PLoS ONE, 15(5), e0232748. https://doi.org/10.1371/journal.pone.0232748
Barca-Mayo, O., Pons-Espinal, M., Follert, P., Armirotti, A., Berdondini, L., & De Pietri Tonelli, D. (2017). Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nature Communications, 8, 14336. https://doi.org/10.1038/ncomms14336
Fernandez, D. C., Komal, R., Langel, J., Ma, J., Duy, P. Q., Penzo, M. A., et al. (2020). Retinal innervation tunes circuits that drive nonphotic entrainment to food. Nature, 581(7807), 194–198. https://doi.org/10.1038/s41586-020-2204-1
Mendoza, J. (2017). Circadian neurons in the lateral habenula: Clocking motivated behaviors. Pharmacology, Biochemistry and Behavior, 162, 55–61. https://doi.org/10.1016/j.pbb.2017.06.013
Son, G. H., Chung, S., & Kim, K. (2011). The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Frontiers in Neuroendocrinology, 32(4), 451–465. https://doi.org/10.1016/j.yfrne.2011.07.003
Jones, J. R., Chaturvedi, S., Granados-Fuentes, D., & Herzog, E. D. (2021). Circadian neurons in the paraventricular nucleus entrain and sustain daily rhythms in glucocorticoids. Nature Communications, 12(1), 5763. https://doi.org/10.1038/s41467-021-25959-9
Koronowski, K. B., & Sassone-Corsi, P. (2021). Communicating clocks shape circadian homeostasis. Science, 371(6530). https://doi.org/10.1126/science.abd0951
Bass, J., & Lazar, M. A. (2016). Circadian time signatures of fitness and disease. Science, 354(6315), 994–999. https://doi.org/10.1126/science.aah4965
Patton, A. P., & Hastings, M. H. (2018). The suprachiasmatic nucleus. Current Biology, 28(15), R816-r822. https://doi.org/10.1016/j.cub.2018.06.052
Maywood, E. S., Smith, E., Hall, S. J., & Hastings, M. H. (1997). A thalamic contribution to arousal-induced, non-photic entrainment of the circadian clock of the Syrian hamster. European Journal of Neuroscience, 9(8), 1739–1747. https://doi.org/10.1111/j.1460-9568.1997.tb01531.x
Filipski, E., King, V. M., Etienne, M. C., Li, X., Claustrat, B., Granda, T. G., et al. (2004). Persistent twenty-four hour changes in liver and bone marrow despite suprachiasmatic nuclei ablation in mice. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 287(4), R844-851. https://doi.org/10.1152/ajpregu.00085.2004
Filipski, E., King, V. M., Li, X., Granda, T. G., Mormont, M. C., Claustrat, B., et al. (2003). Disruption of circadian coordination accelerates malignant growth in mice. Pathologie Biologie, 51(4), 216–219. https://doi.org/10.1016/s0369-8114(03)00034-8
Yamaguchi, S., Isejima, H., Matsuo, T., Okura, R., Yagita, K., Kobayashi, M., et al. (2003). Synchronization of cellular clocks in the suprachiasmatic nucleus. Science, 302(5649), 1408–1412. https://doi.org/10.1126/science.1089287
Maywood, E. S., O’Neill, J. S., Chesham, J. E., & Hastings, M. H. (2007). Minireview: The circadian clockwork of the suprachiasmatic nuclei–analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology, 148(12), 5624–5634. https://doi.org/10.1210/en.2007-0660
Deery, M. J., Maywood, E. S., Chesham, J. E., Sládek, M., Karp, N. A., Green, E. W., et al. (2009). Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Current Biology, 19(23), 2031–2036. https://doi.org/10.1016/j.cub.2009.10.024
Webb, A. B., Angelo, N., Huettner, J. E., & Herzog, E. D. (2009). Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci USA, 106(38), 16493–16498. https://doi.org/10.1073/pnas.0902768106
Harmar, A. J., Marston, H. M., Shen, S., Spratt, C., West, K. M., Sheward, W. J., et al. (2002). The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell, 109(4), 497–508. https://doi.org/10.1016/s0092-8674(02)00736-5
Mazuski, C., Chen, S. P., & Herzog, E. D. (2020). Different roles for VIP neurons in the neonatal and adult suprachiasmatic nucleus. Journal of Biological Rhythms, 35(5), 465–475. https://doi.org/10.1177/0748730420932073
Jones, J. R., Simon, T., Lones, L., & Herzog, E. D. (2018). SCN VIP neurons are essential for normal light-mediated resetting of the circadian system. Journal of Neuroscience, 38(37), 7986–7995. https://doi.org/10.1523/jneurosci.1322-18.2018
Freeman, G. M., Jr., Nakajima, M., Ueda, H. R., & Herzog, E. D. (2013). Picrotoxin dramatically speeds the mammalian circadian clock independent of Cys-loop receptors. Journal of Neurophysiology, 110(1), 103–108. https://doi.org/10.1152/jn.00220.2013
Hermanstyne, T. O., Granados-Fuentes, D., Mellor, R. L., Herzog, E. D., & Nerbonne, J. M. (2017). Acute knockdown of Kv4.1 regulates repetitive firing rates and clock gene expression in the suprachiasmatic nucleus and daily rhythms in locomotor behavior. eNeuro, 4(3). https://doi.org/10.1523/eneuro.0377-16.2017
Granados-Fuentes, D., Hermanstyne, T. O., Carrasquillo, Y., Nerbonne, J. M., & Herzog, E. D. (2015). IA channels encoded by Kv1.4 and Kv4.2 regulate circadian period of PER2 expression in the suprachiasmatic nucleus. J Biol Rhythms, 30(5), 396–407. https://doi.org/10.1177/0748730415593377
Kofuji, P., Mure, L. S., Massman, L. J., Purrier, N., Panda, S., & Engeland, W. C. (2016). Intrinsically photosensitive retinal ganglion cells (ipRGCs) are necessary for light entrainment of peripheral clocks. PLoS ONE, 11(12), e0168651. https://doi.org/10.1371/journal.pone.0168651
Gomez, J. L., Bonaventura, J., Lesniak, W., Mathews, W. B., Sysa-Shah, P., Rodriguez, L. A., et al. (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science, 357(6350), 503–507. https://doi.org/10.1126/science.aan2475
Wang, Y., Jiang, W., Chen, H., Zhou, H., Liu, Z., Liu, Z., et al. (2021). Sympathetic nervous system mediates cardiac remodeling after myocardial infarction in a circadian disruption model. Front Cardiovasc Med, 8, 668387. https://doi.org/10.3389/fcvm.2021.668387
Hablitz, L. M., Gunesch, A. N., Cravetchi, O., Moldavan, M., & Allen, C. N. (2020). Cannabinoid signaling recruits astrocytes to modulate presynaptic function in the suprachiasmatic nucleus. eNeuro, 7(1). https://doi.org/10.1523/eneuro.0081-19.2020
Landgraf, D., Long, J. E., Proulx, C. D., Barandas, R., Malinow, R., & Welsh, D. K. (2016). Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biological Psychiatry, 80(11), 827–835. https://doi.org/10.1016/j.biopsych.2016.03.1050
Tso, C. F., Simon, T., Greenlaw, A. C., Puri, T., Mieda, M., & Herzog, E. D. (2017). Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Current Biology, 27(7), 1055–1061. https://doi.org/10.1016/j.cub.2017.02.037
Sueviriyapan, N., Tso, C. F., Herzog, E. D., & Henson, M. A. (2020). Astrocytic modulation of neuronal activity in the suprachiasmatic nucleus: Insights from mathematical modeling. Journal of Biological Rhythms, 35(3), 287–301. https://doi.org/10.1177/0748730420913672
Kolbe, I., Leinweber, B., Brandenburger, M., & Oster, H. (2019). Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol Metab, 30, 140–151. https://doi.org/10.1016/j.molmet.2019.09.012
Kozlov, S. V., Bogenpohl, J. W., Howell, M. P., Wevrick, R., Panda, S., Hogenesch, J. B., et al. (2007). The imprinted gene Magel2 regulates normal circadian output. Nature Genetics, 39(10), 1266–1272. https://doi.org/10.1038/ng2114
Mizoguchi, A., Takeuchi, T., Himuro, H., Okada, T., & Mizoguchi, E. (2016). Genetically engineered mouse models for studying inflammatory bowel disease. The Journal of Pathology, 238(2), 205–219. https://doi.org/10.1002/path.4640
Annunziato, S., Lutz, C., Henneman, L., Bhin, J., Wong, K., Siteur, B., et al. (2020). In situ CRISPR-Cas9 base editing for the development of genetically engineered mouse models of breast cancer. Embo j, 39(5), e102169. https://doi.org/10.15252/embj.2019102169
Schmid, R. S., Vitucci, M., & Miller, C. R. (2012). Genetically engineered mouse models of diffuse gliomas. Brain Research Bulletin, 88(1), 72–79. https://doi.org/10.1016/j.brainresbull.2011.06.002
Anderson, K. R., Haeussler, M., Watanabe, C., Janakiraman, V., Lund, J., Modrusan, Z., et al. (2018). CRISPR off-target analysis in genetically engineered rats and mice. Nature Methods, 15(7), 512–514. https://doi.org/10.1038/s41592-018-0011-5
Korge, S., Grudziecki, A., & Kramer, A. (2015). Highly efficient genome editing via CRISPR/Cas9 to create clock gene knockout cells. Journal of Biological Rhythms, 30(5), 389–395. https://doi.org/10.1177/0748730415597519
Kersten, K., de Visser, K. E., van Miltenburg, M. H., & Jonkers, J. (2017). Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med, 9(2), 137–153. https://doi.org/10.15252/emmm.201606857
Haque, S. N., Booreddy, S. R., & Welsh, D. K. (2019). Effects of BMAL1 manipulation on the brain’s master circadian clock and behavior. The Yale Journal of Biology and Medicine, 92(2), 251–258.
Zhang, Y., Devocelle, A., Desterke, C., de Souza, L. E. B., Hadadi, É., Acloque, H., et al. (2021). BMAL1 Knockdown leans epithelial-mesenchymal balance toward epithelial properties and decreases the chemoresistance of colon carcinoma cells. Int J Mol Sci, 22(10). https://doi.org/10.3390/ijms22105247
Liu, X., Xiao, W., Jiang, Y., Zou, L., Chen, F., Xiao, W., et al. (2021). Bmal1 regulates the redox rhythm of HSPB1, and homooxidized HSPB1 attenuates the oxidative stress injury of cardiomyocytes. Oxidative Medicine and Cellular Longevity, 2021, 5542815. https://doi.org/10.1155/2021/5542815
Umemura, A., He, F., Taniguchi, K., Nakagawa, H., Yamachika, S., Font-Burgada, J., et al. (2016). p62, Upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell, 29(6), 935–948. https://doi.org/10.1016/j.ccell.2016.04.006
Liu, Z., Selby, C. P., Yang, Y., Lindsey-Boltz, L. A., Cao, X., Eynullazada, K., et al. (2020). Circadian regulation of c-MYC in mice. Proc Natl Acad Sci U S A, 117(35), 21609–21617. https://doi.org/10.1073/pnas.2011225117
Lee, S., Donehower, L. A., Herron, A. J., Moore, D. D., & Fu, L. (2010). Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE, 5(6), e10995. https://doi.org/10.1371/journal.pone.0010995
Papagiannakopoulos, T., Bauer, M. R., Davidson, S. M., Heimann, M., Subbaraj, L., Bhutkar, A., et al. (2016). Circadian rhythm disruption promotes lung tumorigenesis. Cell Metabolism, 24(2), 324–331. https://doi.org/10.1016/j.cmet.2016.07.001
Liu, J. L., Wang, C. Y., Cheng, T. Y., Rixiati, Y., Ji, C., Deng, M., et al. (2021). Circadian clock disruption suppresses PDL1(+) intraepithelial B cells in experimental colitis and colitis-associated colorectal cancer. Cellular and Molecular Gastroenterology and Hepatology, 12(1), 251–276. https://doi.org/10.1016/j.jcmgh.2021.02.008
Pan, X., Bradfield, C. A., & Hussain, M. M. (2016). Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nature Communications, 7, 13011. https://doi.org/10.1038/ncomms13011
Kettner, N. M., Voicu, H., Finegold, M. J., Coarfa, C., Sreekumar, A., Putluri, N., et al. (2016). Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell, 30(6), 909–924. https://doi.org/10.1016/j.ccell.2016.10.007
Alexander, R. K., Liou, Y. H., Knudsen, N. H., Starost, K. A., Xu, C., Hyde, A. L., et al. (2020). Bmal1 integrates mitochondrial metabolism and macrophage activation. Elife, 9. https://doi.org/10.7554/eLife.54090
Zeng, Z. L., Wu, M. W., Sun, J., Sun, Y. L., Cai, Y. C., Huang, Y. J., et al. (2010). Effects of the biological clock gene Bmal1 on tumour growth and anti-cancer drug activity. Journal of Biochemistry, 148(3), 319–326. https://doi.org/10.1093/jb/mvq069
Zeng, Z. L., Luo, H. Y., Yang, J., Wu, W. J., Chen, D. L., Huang, P., et al. (2014). Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clincal Cancer Research, 20(4), 1042–1052. https://doi.org/10.1158/1078-0432.Ccr-13-0171
Puram, R. V., Kowalczyk, M. S., de Boer, C. G., Schneider, R. K., Miller, P. G., McConkey, M., et al. (2016). Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell, 165(2), 303–316. https://doi.org/10.1016/j.cell.2016.03.015
Korkmaz, T., Aygenli, F., Emisoglu, H., Ozcelik, G., Canturk, A., Yilmaz, S., et al. (2018). Opposite carcinogenic effects of circadian clock gene BMAL1. Science and Reports, 8(1), 16023. https://doi.org/10.1038/s41598-018-34433-4
Wang, Y., Sun, N., Lu, C., Bei, Y., Qian, R., & Hua, L. (2017). Upregulation of circadian gene ‘hClock’ contribution to metastasis of colorectal cancer. International Journal of Oncology, 50(6), 2191–2199. https://doi.org/10.3892/ijo.2017.3987
Li, X. M., Claustrat, B., Hastings, M. H., Albrecht, U., & Lévi, F. (2007). Interactions between clock gene mutation, circadian phenotype and tumor growth in mice. Pathologie Biologie, 55(3–4), 194–197. https://doi.org/10.1016/j.patbio.2006.12.003
Yang, X., Wood, P. A., Ansell, C. M., Quiton, D. F., Oh, E. Y., Du-Quiton, J., et al. (2009). The circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiology International, 26(7), 1323–1339. https://doi.org/10.3109/07420520903431301
Yang, G., Yang, Y., Tang, H., & Yang, K. (2020). Loss of the clock gene Per1 promotes oral squamous cell carcinoma progression via the AKT/mTOR pathway. Cancer Science, 111(5), 1542–1554. https://doi.org/10.1111/cas.14362
Han, Y., Meng, F., Venter, J., Wu, N., Wan, Y., Standeford, H., et al. (2016). miR-34a-dependent overexpression of Per1 decreases cholangiocarcinoma growth. Journal of Hepatology, 64(6), 1295–1304. https://doi.org/10.1016/j.jhep.2016.02.024
Fu, L., Pelicano, H., Liu, J., Huang, P., & Lee, C. (2002). The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell, 111(1), 41–50. https://doi.org/10.1016/s0092-8674(02)00961-3
Wood, P. A., Yang, X., Taber, A., Oh, E. Y., Ansell, C., Ayers, S. E., et al. (2008). Period 2 mutation accelerates ApcMin/+ tumorigenesis. Molecular Cancer Research, 6(11), 1786–1793. https://doi.org/10.1158/1541-7786.Mcr-08-0196
Yang, X., Wood, P. A., Oh, E. Y., Du-Quiton, J., Ansell, C. M., & Hrushesky, W. J. (2009). Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Research and Treatment, 117(2), 423–431. https://doi.org/10.1007/s10549-008-0133-z
Ma, D., Hou, L., Xia, H., Li, H., Fan, H., Jia, X., et al. (2020). PER2 inhibits proliferation and stemness of glioma stem cells via the Wnt/β-catenin signaling pathway. Oncology Reports, 44(2), 533–542. https://doi.org/10.3892/or.2020.7624
Hua, H., Wang, Y., Wan, C., Liu, Y., Zhu, B., Yang, C., et al. (2006). Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Science, 97(7), 589–596. https://doi.org/10.1111/j.1349-7006.2006.00225.x
Hua, H., Wang, Y., Wan, C., Liu, Y., Zhu, B., Wang, X., et al. (2007). Inhibition of tumorigenesis by intratumoral delivery of the circadian gene mPer2 in C57BL/6 mice. Cancer Gene Therapy, 14(9), 815–818. https://doi.org/10.1038/sj.cgt.7701061
Katamune, C., Koyanagi, S., Hashikawa, K. I., Kusunose, N., Akamine, T., Matsunaga, N., et al. (2019). Mutation of the gene encoding the circadian clock component PERIOD2 in oncogenic cells confers chemoresistance by up-regulating the Aldh3a1 gene. Journal of Biological Chemistry, 294(2), 547–558. https://doi.org/10.1074/jbc.RA118.004942
Gu, X., Xing, L., Shi, G., Liu, Z., Wang, X., Qu, Z., et al. (2012). The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death and Differentiation, 19(3), 397–405. https://doi.org/10.1038/cdd.2011.103
Matsumura, R., & Akashi, M. (2019). Role of the clock gene Period3 in the human cell-autonomous circadian clock. Genes to Cells, 24(2), 162–171. https://doi.org/10.1111/gtc.12664
Bae, K., Jin, X., Maywood, E. S., Hastings, M. H., Reppert, S. M., & Weaver, D. R. (2001). Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron, 30(2), 525–536. https://doi.org/10.1016/s0896-6273(01)00302-6
Gauger, M. A., & Sancar, A. (2005). Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Research, 65(15), 6828–6834. https://doi.org/10.1158/0008-5472.Can-05-1119
Mteyrek, A., Filipski, E., Guettier, C., Oklejewicz, M., van der Horst, G. T., Okyar, A., et al. (2017). Critical cholangiocarcinogenesis control by cryptochrome clock genes. International Journal of Cancer, 140(11), 2473–2483. https://doi.org/10.1002/ijc.30663
Ozturk, N., Lee, J. H., Gaddameedhi, S., & Sancar, A. (2009). Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc Natl Acad Sci USA, 106(8), 2841–2846. https://doi.org/10.1073/pnas.0813028106
Kinouchi, K., & Sassone-Corsi, P. (2020). Metabolic rivalry: Circadian homeostasis and tumorigenesis. Nature Reviews Cancer, 20(11), 645–661. https://doi.org/10.1038/s41568-020-0291-9
Vajtay, T. J., St Thomas, J. J., Takacs, T. E., McGann, E. G., & Weber, E. T. (2017). Duration and timing of daily light exposure influence the rapid shifting of BALB/cJ mouse circadian locomotor rhythms. Physiology & Behavior, 179, 200–207. https://doi.org/10.1016/j.physbeh.2017.06.010
Casiraghi, L. P., Oda, G. A., Chiesa, J. J., Friesen, W. O., & Golombek, D. A. (2012). Forced desynchronization of activity rhythms in a model of chronic jet lag in mice. Journal of Biological Rhythms, 27(1), 59–69. https://doi.org/10.1177/0748730411429447
Horsey, E. A., Maletta, T., Turner, H., Cole, C., Lehmann, H., & Fournier, N. M. (2019). Chronic jet lag simulation decreases hippocampal neurogenesis and enhances depressive behaviors and cognitive deficits in adult male rats. Frontiers in Behavioral Neuroscience, 13, 272. https://doi.org/10.3389/fnbeh.2019.00272
Woller, A., & Gonze, D. (2021). Circadian misalignment and metabolic disorders: A story of twisted clocks. Biology (Basel), 10(3) https://doi.org/10.3390/biology10030207
Hadadi, E., Taylor, W., Li, X. M., Aslan, Y., Villote, M., Rivière, J., et al. (2020). Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nature Communications, 11(1), 3193. https://doi.org/10.1038/s41467-020-16890-6
Van Dycke, K. C., Rodenburg, W., van Oostrom, C. T., van Kerkhof, L. W., Pennings, J. L., Roenneberg, T., et al. (2015). Chronically alternating light cycles increase breast cancer risk in mice. Current Biology, 25(14), 1932–1937. https://doi.org/10.1016/j.cub.2015.06.012
Wu, M., Zeng, J., Chen, Y., Zeng, Z., Zhang, J., Cai, Y., et al. (2012). Experimental chronic jet lag promotes growth and lung metastasis of Lewis lung carcinoma in C57BL/6 mice. Oncology Reports, 27(5), 1417–1428. https://doi.org/10.3892/or.2012.1688
Filipski, E., Innominato, P. F., Wu, M., Li, X. M., Iacobelli, S., Xian, L. J., et al. (2005). Effects of light and food schedules on liver and tumor molecular clocks in mice. Journal of the National Cancer Institute, 97(7), 507–517. https://doi.org/10.1093/jnci/dji083
Filipski, E., & Lévi, F. (2009). Circadian disruption in experimental cancer processes. Integrative Cancer Therapies, 8(4), 298–302. https://doi.org/10.1177/1534735409352085
Aiello, I., Fedele, M. L. M., Román, F., Marpegan, L., Caldart, C., Chiesa, J. J., et al. (2020). Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci Adv, 6(42). https://doi.org/10.1126/sciadv.aaz4530
Logan, R. W., Zhang, C., Murugan, S., O’Connell, S., Levitt, D., Rosenwasser, A. M., et al. (2012). Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. The Journal of Immunology, 188(6), 2583–2591. https://doi.org/10.4049/jimmunol.1102715
Zeng, X., Liang, C., & Yao, J. (2020). Chronic shift-lag promotes NK cell ageing and impairs immunosurveillance in mice by decreasing the expression of CD122. Journal of Cellular and Molecular Medicine, 24(24), 14583–14595. https://doi.org/10.1111/jcmm.16088
Cos, S., Mediavilla, D., Martinez-Campa, C., Gonzalez, A., Alonso-Gonzalez, C., & Sanchez-Barcelo, E. J. (2006). Exposure to light-at-night increases the growth of DMBA-induced mammary adenocarcinomas in rats. Cancer Letters, 235(2), 266–271. https://doi.org/10.1016/j.canlet.2005.04.025
Yasuniwa, Y., Izumi, H., Wang, K. Y., Shimajiri, S., Sasaguri, Y., Kawai, K., et al. (2010). Circadian disruption accelerates tumor growth and angio/stromagenesis through a Wnt signaling pathway. PLoS ONE, 5(12), e15330. https://doi.org/10.1371/journal.pone.0015330
Vinogradova, I. A., Anisimov, V. N., Bukalev, A. V., Semenchenko, A. V., & Zabezhinski, M. A. (2009). Circadian disruption induced by light-at-tnight accelerates aging and promotes tumorigenesis in rats. Aging (Albany NY), 1(10), 855–865. https://doi.org/10.18632/aging.100092
Blask, D. E., Dauchy, R. T., Dauchy, E. M., Mao, L., Hill, S. M., Greene, M. W., et al. (2014). Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE, 9(8), e102776. https://doi.org/10.1371/journal.pone.0102776
Oh, E. Y., Yang, X., Friedman, A., Ansell, C. M., Du-Quiton, J., Quiton, D. F., et al. (2010). Circadian transcription profile of mouse breast cancer under light-dark and dark-dark conditions. Cancer Genomics & Proteomics, 7(6), 311–322.
Xiang, S., Dauchy, R. T., Hauch, A., Mao, L., Yuan, L., Wren, M. A., et al. (2015). Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. Journal of Pineal Research, 59(1), 60–69. https://doi.org/10.1111/jpi.12239
Bishehsari, F., Saadalla, A., Khazaie, K., Engen, P. A., Voigt, R. M., Shetuni, B. B., et al. (2016). Light/dark shifting promotes alcohol-induced colon carcinogenesis: Possible role of intestinal inflammatory milieu and microbiota. Int J Mol Sci, 17(12). https://doi.org/10.3390/ijms17122017
Tahara, Y., & Shibata, S. (2018). Entrainment of the mouse circadian clock: Effects of stress, exercise, and nutrition. Free Radical Biology & Medicine, 119, 129–138. https://doi.org/10.1016/j.freeradbiomed.2017.12.026
Kahleova, H., Lloren, J. I., Mashchak, A., Hill, M., & Fraser, G. E. (2017). Meal frequency and timing are associated with changes in body mass index in adventist health study 2. Journal of Nutrition, 147(9), 1722–1728. https://doi.org/10.3945/jn.116.244749
Kogevinas, M., Espinosa, A., Castelló, A., Gómez-Acebo, I., Guevara, M., Martin, V., et al. (2018). Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study). International Journal of Cancer, 143(10), 2380–2389. https://doi.org/10.1002/ijc.31649
Marinac, C. R., Natarajan, L., Sears, D. D., Gallo, L. C., Hartman, S. J., Arredondo, E., et al. (2015). Prolonged nightly fasting and breast cancer risk: Findings from NHANES (2009–2010). Cancer Epidemiology, Biomarkers & Prevention, 24(5), 783–789. https://doi.org/10.1158/1055-9965.Epi-14-1292
Bishehsari, F., Engen, P. A., Voigt, R. M., Swanson, G., Shaikh, M., Wilber, S., et al. (2020). Abnormal eating patterns cause circadian disruption and promote alcohol-associated colon carcinogenesis. Cellular and Molecular Gastroenterology and Hepatology, 9(2), 219–237. https://doi.org/10.1016/j.jcmgh.2019.10.011
Bishehsari, F., Preuss, F., Mirbagheri, S. S., Zhang, L., Shaikh, M., & Keshavarzian, A. (2020). Interaction of alcohol with time of eating on markers of circadian dyssynchrony and colon tissue injury. Chemico-Biological Interactions, 325, 109132. https://doi.org/10.1016/j.cbi.2020.109132
Bishehsari, F., Engen, P. A., Adnan, D., Sarrafi, S., Wilber, S., Shaikh, M., et al. (2021). Abnormal food timing and predisposition to weight gain: Role of barrier dysfunction and microbiota. Translational Research, 231, 113–123. https://doi.org/10.1016/j.trsl.2020.11.007
Eckel-Mahan, K. L., Patel, V. R., de Mateo, S., Orozco-Solis, R., Ceglia, N. J., Sahar, S., et al. (2013). Reprogramming of the circadian clock by nutritional challenge. Cell, 155(7), 1464–1478. https://doi.org/10.1016/j.cell.2013.11.034
Potter, G. D., Cade, J. E., Grant, P. J., & Hardie, L. J. (2016). Nutrition and the circadian system. British Journal of Nutrition, 116(3), 434–442. https://doi.org/10.1017/s0007114516002117
Sundaram, S., & Yan, L. (2018). Time-restricted feeding mitigates high-fat diet-enhanced mammary tumorigenesis in MMTV-PyMT mice. Nutrition Research, 59, 72–79. https://doi.org/10.1016/j.nutres.2018.07.014
Yan, L., Sundaram, S., Mehus, A. A., & Picklo, M. J. (2019). Time-restricted feeding attenuates high-fat diet-enhanced spontaneous metastasis of Lewis lung carcinoma in mice. Anticancer Res, 39(4), 1739–1748. https://doi.org/10.21873/anticanres.13280
Yan, L., Rust, B. M., & Picklo, M. J. (2020). Plasma metabolomic changes in mice with time-restricted feeding-attenuated spontaneous metastasis of Lewis lung carcinoma. Anticancer Res, 40(4), 1833–1841. https://doi.org/10.21873/anticanres.14137
Das, M., Ellies, L. G., Kumar, D., Sauceda, C., Oberg, A., Gross, E., et al. (2021). Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nature Communications, 12(1), 565. https://doi.org/10.1038/s41467-020-20743-7
Molina-Aguilar, C., Guerrero-Carrillo, M. J., Espinosa-Aguirre, J. J., Olguin-Reyes, S., Castro-Belio, T., Vázquez-Martínez, O., et al. (2017). Time-caloric restriction inhibits the neoplastic transformation of cirrhotic liver in rats treated with diethylnitrosamine. Carcinogenesis, 38(8), 847–858. https://doi.org/10.1093/carcin/bgx052
Tahara, Y., Aoyama, S., & Shibata, S. (2017). The mammalian circadian clock and its entrainment by stress and exercise. The Journal of Physiological Sciences, 67(1), 1–10. https://doi.org/10.1007/s12576-016-0450-7
Tahara, Y., Shiraishi, T., Kikuchi, Y., Haraguchi, A., Kuriki, D., Sasaki, H., et al. (2015). Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Science and Reports, 5, 11417. https://doi.org/10.1038/srep11417
Sasaki, H., Hattori, Y., Ikeda, Y., Kamagata, M., Iwami, S., Yasuda, S., et al. (2016). Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Science and Reports, 6, 27607. https://doi.org/10.1038/srep27607
Spiliopoulou, P., Gavriatopoulou, M., Kastritis, E., Dimopoulos, M. A., & Terzis, G. (2021). Exercise-induced changes in tumor growth via tumor immunity. Sports (Basel), 9(4). https://doi.org/10.3390/sports9040046
Rundqvist, H., Velica, P., Barbieri, L., Gameiro, P. A., Bargiela, D., Gojkovic, M., et al. (2020). Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife, 9. https://doi.org/10.7554/eLife.59996
Hoevenaar-Blom, M. P., Spijkerman, A. M., Kromhout, D., van den Berg, J. F., & Verschuren, W. M. (2011). Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: The MORGEN study. Sleep, 34(11), 1487–1492. https://doi.org/10.5665/sleep.1382
Suchecki, D., & Tufik, S. (2000). Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiology & Behavior, 68(3), 309–316. https://doi.org/10.1016/s0031-9384(99)00181-x
De Lorenzo, B. H., de Oliveira Marchioro, L., Greco, C. R., & Suchecki, D. (2015). Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology, 57, 134–143. https://doi.org/10.1016/j.psyneuen.2015.04.006
Sousa, M. E. P., Gonzatti, M. B., Fernandes, E. R., Freire, B. M., Guereschi, M. G., Basso, A. S., et al. (2020). Invariant natural killer T cells resilience to paradoxical sleep deprivation-associated stress. Brain, Behavior, and Immunity, 90, 208–215. https://doi.org/10.1016/j.bbi.2020.08.018
Zielinski, M. R., Davis, J. M., Fadel, J. R., & Youngstedt, S. D. (2012). Influence of chronic moderate sleep restriction and exercise on inflammation and carcinogenesis in mice. Brain, Behavior, and Immunity, 26(4), 672–679. https://doi.org/10.1016/j.bbi.2012.03.002
Huang, J., Song, P., Hang, K., Chen, Z., Zhu, Z., Zhang, Y., et al. (2021). Sleep deprivation disturbs immune surveillance and promotes the progression of hepatocellular carcinoma. Frontiers in Immunology, 12, 727959. https://doi.org/10.3389/fimmu.2021.727959
De Lorenzo, B. H. P., Novaes, E. B. R. R., Paslar Leal, T., Piqueira Garcia, N., Martins Dos Santos, R. M., Alvares-Saraiva, A. M., et al. (2018). Chronic sleep restriction impairs the antitumor immune response in mice. NeuroImmunoModulation, 25(2), 59–67. https://doi.org/10.1159/000490352
Kaushal, N., Ramesh, V., & Gozal, D. (2012). Human apolipoprotein E4 targeted replacement in mice reveals increased susceptibility to sleep disruption and intermittent hypoxia. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 303(1), R19-29. https://doi.org/10.1152/ajpregu.00025.2012
Hakim, F., Wang, Y., Zhang, S. X., Zheng, J., Yolcu, E. S., Carreras, A., et al. (2014). Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Research, 74(5), 1329–1337. https://doi.org/10.1158/0008-5472.Can-13-3014
Capri, K. M., Maroni, M. J., Deane, H. V., Concepcion, H. A., DeCourcey, H., Logan, R. W., et al. (2019). Male C57BL6/N and C57BL6/J mice respond differently to constant light and running-wheel access. Frontiers in Behavioral Neuroscience, 13, 268. https://doi.org/10.3389/fnbeh.2019.00268
Khroyan, T. V., Zhang, J., Yang, L., Zou, B., Xie, J., Pascual, C., et al. (2012). Rodent motor and neuropsychological behaviour measured in home cages using the integrated modular platform SmartCage™. Clinical and Experimental Pharmacology and Physiology, 39(7), 614–622. https://doi.org/10.1111/j.1440-1681.2012.05719.x
Hicks, J. A., Hatzidis, A., Arruda, N. L., Gelineau, R. R., De Pina, I. M., Adams, K. W., et al. (2016). Voluntary wheel-running attenuates insulin and weight gain and affects anxiety-like behaviors in C57BL6/J mice exposed to a high-fat diet. Behavioural Brain Research, 310, 1–10. https://doi.org/10.1016/j.bbr.2016.04.051
Bevins, R. A., & Besheer, J. (2006). Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory.’ Nature Protocols, 1(3), 1306–1311. https://doi.org/10.1038/nprot.2006.205
McCarthy, M. J., & Welsh, D. K. (2012). Cellular circadian clocks in mood disorders. Journal of Biological Rhythms, 27(5), 339–352. https://doi.org/10.1177/0748730412456367
Filipski, E., Delaunay, F., King, V. M., Wu, M. W., Claustrat, B., Gréchez-Cassiau, A., et al. (2004). Effects of chronic jet lag on tumor progression in mice. Cancer Research, 64(21), 7879–7885. https://doi.org/10.1158/0008-5472.Can-04-0674
Shimizu, K., Iyoda, T., Okada, M., Yamasaki, S., & Fujii, S. I. (2018). Immune suppression and reversal of the suppressive tumor microenvironment. International Immunology, 30(10), 445–454. https://doi.org/10.1093/intimm/dxy042
Wang, Y., Ding, Y., Guo, N., & Wang, S. (2019). MDSCs: Key criminals of tumor pre-metastatic niche formation. Frontiers in Immunology, 10, 172. https://doi.org/10.3389/fimmu.2019.00172
Nguyen, K. D., Fentress, S. J., Qiu, Y., Yun, K., Cox, J. S., & Chawla, A. (2013). Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science, 341(6153), 1483–1488. https://doi.org/10.1126/science.1240636
Silver, A. C., Arjona, A., Hughes, M. E., Nitabach, M. N., & Fikrig, E. (2012). Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain, Behavior, and Immunity, 26(3), 407–413. https://doi.org/10.1016/j.bbi.2011.10.001
Ella, K., Csépányi-Kömi, R., & Káldi, K. (2016). Circadian regulation of human peripheral neutrophils. Brain, Behavior, and Immunity, 57, 209–221. https://doi.org/10.1016/j.bbi.2016.04.016
Baumann, A., Gönnenwein, S., Bischoff, S. C., Sherman, H., Chapnik, N., Froy, O., et al. (2013). The circadian clock is functional in eosinophils and mast cells. Immunology, 140(4), 465–474. https://doi.org/10.1111/imm.12157
Hemmers, S., & Rudensky, A. Y. (2015). The cell-intrinsic circadian clock is dispensable for lymphocyte differentiation and function. Cell Reports, 11(9), 1339–1349. https://doi.org/10.1016/j.celrep.2015.04.058
Scheiermann, C., Gibbs, J., Ince, L., & Loudon, A. (2018). Clocking in to immunity. Nature Reviews Immunology, 18(7), 423–437. https://doi.org/10.1038/s41577-018-0008-4
de Assis, L. V. M., Kinker, G. S., Moraes, M. N., Markus, R. P., Fernandes, P. A., & Castrucci, A. M. L. (2018). Expression of the circadian clock gene BMAL1 positively correlates with antitumor immunity and patient survival in metastatic melanoma. Frontiers in Oncology, 8, 185. https://doi.org/10.3389/fonc.2018.00185
Wang, X., Li, Y., Fu, J., Zhou, K., & Wang, T. (2021). ARNTL2 is a prognostic biomarker and correlates with immune cell infiltration in triple-negative breast cancer. Pharmgenomics Pers Med, 14, 1425–1440. https://doi.org/10.2147/pgpm.S331431
Curtis, A. M., Fagundes, C. T., Yang, G., Palsson-McDermott, E. M., Wochal, P., McGettrick, A. F., et al. (2015). Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A, 112(23), 7231–7236. https://doi.org/10.1073/pnas.1501327112
He, F., Ru, X., & Wen, T. (2020). NRF2, a transcription factor for stress response and beyond. Int J Mol Sci, 21(13). https://doi.org/10.3390/ijms21134777
Gowda, P., Lathoria, K., Sharma, S., Patrick, S., Umdor, S. B., & Sen, E. (2021). Rewiring of lactate-Interleukin-1β autoregulatory loop with clock-Bmal1: A feed-forward circuit in glioma. Molecular and Cellular Biology, 41(9), e0044920. https://doi.org/10.1128/mcb.00449-20
Early, J. O., Menon, D., Wyse, C. A., Cervantes-Silva, M. P., Zaslona, Z., Carroll, R. G., et al. (2018). Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc Natl Acad Sci U S A, 115(36), E8460-e8468. https://doi.org/10.1073/pnas.1800431115
Shaashua, L., Mayer, S., Lior, C., Lavon, H., Novoselsky, A., & Scherz-Shouval, R. (2020). Stromal expression of the core clock gene period 2 is essential for tumor initiation and metastatic colonization. Front Cell Dev Biol, 8, 587697. https://doi.org/10.3389/fcell.2020.587697
Lee, I. K., Song, H., Kim, H., Kim, I. S., Tran, N. L., Kim, S. H., et al. (2020). RORα regulates cholesterol metabolism of CD8(+) T cells for anticancer immunity. Cancers (Basel), 12(7). https://doi.org/10.3390/cancers12071733
Sato, S., Sakurai, T., Ogasawara, J., Takahashi, M., Izawa, T., Imaizumi, K., et al. (2014). A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. The Journal of Immunology, 192(1), 407–417. https://doi.org/10.4049/jimmunol.1301982
Lam, M. T., Cho, H., Lesch, H. P., Gosselin, D., Heinz, S., Tanaka-Oishi, Y., et al. (2013). Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature, 498(7455), 511–515. https://doi.org/10.1038/nature12209
Hams, E., Roberts, J., Bermingham, R., & Fallon, P. G. (2021). Functions for retinoic acid-related orphan receptor alpha (RORα) in the activation of macrophages during lipopolysaccharide-induced septic shock. Frontiers in Immunology, 12, 647329. https://doi.org/10.3389/fimmu.2021.647329
Yu, X., Rollins, D., Ruhn, K. A., Stubblefield, J. J., Green, C. B., Kashiwada, M., et al. (2013). TH17 cell differentiation is regulated by the circadian clock. Science, 342(6159), 727–730. https://doi.org/10.1126/science.1243884
Hu, X., Liu, X., Moisan, J., Wang, Y., Lesch, C. A., Spooner, C., et al. (2016). Synthetic RORγ agonists regulate multiple pathways to enhance antitumor immunity. Oncoimmunology, 5(12), e1254854. https://doi.org/10.1080/2162402x.2016.1254854
Perfilyeva, Y. V., Abdolla, N., Ostapchuk, Y. O., Tleulieva, R., Krasnoshtanov, V. C., & Belyaev, N. N. (2017). Expansion of CD11b(+)Ly6G(high) and CD11b(+)CD49d(+) myeloid cells with suppressive potential in mice with chronic inflammation and light-at-night-induced circadian disruption. Inflammation Research, 66(8), 711–724. https://doi.org/10.1007/s00011-017-1052-4
Ebihara, S., Marks, T., Hudson, D. J., & Menaker, M. (1986). Genetic control of melatonin synthesis in the pineal gland of the mouse. Science, 231(4737), 491–493. https://doi.org/10.1126/science.3941912
Cheon, D. J., & Orsulic, S. (2011). Mouse models of cancer. Annual Review of Pathology: Mechanisms of Disease, 6, 95–119. https://doi.org/10.1146/annurev.pathol.3.121806.154244
Maser, R. S., Choudhury, B., Campbell, P. J., Feng, B., Wong, K. K., Protopopov, A., et al. (2007). Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature, 447(7147), 966–971. https://doi.org/10.1038/nature05886
Jennings, C. G., Landman, R., Zhou, Y., Sharma, J., Hyman, J., Movshon, J. A., et al. (2016). Opportunities and challenges in modeling human brain disorders in transgenic primates. Nature Neuroscience, 19(9), 1123–1130. https://doi.org/10.1038/nn.4362
Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature, 496(7446), 498–503. https://doi.org/10.1038/nature12111
White, R., Rose, K., & Zon, L. (2013). Zebrafish cancer: The state of the art and the path forward. Nature Reviews Cancer, 13(9), 624–636. https://doi.org/10.1038/nrc3589
Basti, A., Fior, R., Yalҫin, M., Póvoa, V., Astaburuaga, R., Li, Y., et al. (2020). The core-clock gene NR1D1 impacts cell motility in vitro and invasiveness in a zebrafish xenograft colon cancer model. Cancers (Basel), 12(4). https://doi.org/10.3390/cancers12040853
Hamilton, N., Diaz-de-Cerio, N., & Whitmore, D. (2015). Impaired light detection of the circadian clock in a zebrafish melanoma model. Cell Cycle, 14(8), 1232–1241. https://doi.org/10.1080/15384101.2015.1014146
Mure, L. S., Le, H. D., Benegiamo, G., Chang, M. W., Rios, L., Jillani, N., et al. (2018). Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science, 359(6381). https://doi.org/10.1126/science.aao0318
Qiu, P., Jiang, J., Liu, Z., Cai, Y., Huang, T., Wang, Y., et al. (2019). BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. National Science Review, 6(1), 87–100. https://doi.org/10.1093/nsr/nwz002
Mukherji, A., Bailey, S. M., Staels, B., & Baumert, T. F. (2019). The circadian clock and liver function in health and disease. Journal of Hepatology, 71(1), 200–211. https://doi.org/10.1016/j.jhep.2019.03.020
Takahashi, J. S. (2017). Transcriptional architecture of the mammalian circadian clock. Nature Reviews Genetics, 18(3), 164–179. https://doi.org/10.1038/nrg.2016.150
Tao, L., Yu, H., Liang, R., Jia, R., Wang, J., Jiang, K., et al. (2019). Rev-erbα inhibits proliferation by reducing glycolytic flux and pentose phosphate pathway in human gastric cancer cells. Oncogenesis, 8(10), 57. https://doi.org/10.1038/s41389-019-0168-5
Wang, X., Wang, N., Wei, X., Yu, H., & Wang, Z. (2018). REV-ERBα reduction is associated with clinicopathological features and prognosis in human gastric cancer. Oncology Letters, 16(2), 1499–1506. https://doi.org/10.3892/ol.2018.8809
Sulli, G., Rommel, A., Wang, X., Kolar, M. J., Puca, F., Saghatelian, A., et al. (2018). Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature, 553(7688), 351–355. https://doi.org/10.1038/nature25170
Dong, Z., Zhang, G., Qu, M., Gimple, R. C., Wu, Q., Qiu, Z., et al. (2019). Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discovery, 9(11), 1556–1573. https://doi.org/10.1158/2159-8290.Cd-19-0215
Shen, W., Zhang, W., Ye, W., Wang, H., Zhang, Q., Shen, J., et al. (2020). SR9009 induces a REV-ERB dependent anti-small-cell lung cancer effect through inhibition of autophagy. Theranostics, 10(10), 4466–4480. https://doi.org/10.7150/thno.42478
Wang, Y., Kojetin, D., & Burris, T. P. (2015). Anti-proliferative actions of a synthetic REV-ERBα/β agonist in breast cancer cells. Biochemical Pharmacology, 96(4), 315–322. https://doi.org/10.1016/j.bcp.2015.06.010
Trump, R. P., Bresciani, S., Cooper, A. W., Tellam, J. P., Wojno, J., Blaikley, J., et al. (2013). Optimized chemical probes for REV-ERBα. Journal of Medicinal Chemistry, 56(11), 4729–4737. https://doi.org/10.1021/jm400458q
Chen, Z., Yoo, S. H., & Takahashi, J. S. (2013). Small molecule modifiers of circadian clocks. Cellular and Molecular Life Sciences, 70(16), 2985–2998. https://doi.org/10.1007/s00018-012-1207-y
He, B., Nohara, K., Park, N., Park, Y. S., Guillory, B., Zhao, Z., et al. (2016). The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metabolism, 23(4), 610–621. https://doi.org/10.1016/j.cmet.2016.03.007
Goh, J. X. H., Tan, L. T., Goh, J. K., Chan, K. G., Pusparajah, P., Lee, L. H., et al. (2019). Nobiletin and derivatives: Functional compounds from citrus fruit peel for colon cancer chemoprevention. Cancers (Basel), 11(6). https://doi.org/10.3390/cancers11060867
Turdo, A., Glaviano, A., Pepe, G., Calapà, F., Raimondo, S., Fiori, M. E., et al. (2021). Nobiletin and xanthohumol sensitize colorectal cancer stem cells to standard chemotherapy. Cancers (Basel), 13(16). https://doi.org/10.3390/cancers13163927
Guney Eskiler, G., Deveci, A. O., Bilir, C., & Kaleli, S. (2019). Synergistic effects of nobiletin and sorafenib combination on metastatic prostate cancer cells. Nutrition and Cancer, 71(8), 1299–1312. https://doi.org/10.1080/01635581.2019.1601237
Moon, J. Y., Cho, M., Ahn, K. S., & Cho, S. K. (2013). Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutrition and Cancer, 65(2), 286–295. https://doi.org/10.1080/01635581.2013.756529
Feng, S. L., Tian, Y., Huo, S., Qu, B., Liu, R. M., Xu, P., et al. (2020). Nobiletin potentiates paclitaxel anticancer efficacy in A549/T xenograft model: Pharmacokinetic and pharmacological study. Phytomedicine, 67, 153141. https://doi.org/10.1016/j.phymed.2019.153141
Uesato, S., Yamashita, H., Maeda, R., Hirata, Y., Yamamoto, M., Matsue, S., et al. (2014). Synergistic antitumor effect of a combination of paclitaxel and carboplatin with nobiletin from Citrus depressa on non-small-cell lung cancer cell lines. Planta Medica, 80(6), 452–457. https://doi.org/10.1055/s-0034-1368321
Wu, X., Song, M., Qiu, P., Rakariyatham, K., Li, F., Gao, Z., et al. (2017). Synergistic chemopreventive effects of nobiletin and atorvastatin on colon carcinogenesis. Carcinogenesis, 38(4), 455–464. https://doi.org/10.1093/carcin/bgx018
Li, N., Zhang, Z., Jiang, G., Sun, H., & Yu, D. (2019). Nobiletin sensitizes colorectal cancer cells to oxaliplatin by PI3K/Akt/MTOR pathway. Front Biosci (Landmark Ed), 24(2), 303–312. https://doi.org/10.2741/4719
Yang, J., Yang, Y., Wang, L., Jin, Q., & Pan, M. (2020). Nobiletin selectively inhibits oral cancer cell growth by promoting apoptosis and DNA damage in vitro. Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology, 130(4), 419–427. https://doi.org/10.1016/j.oooo.2020.06.020
Liu, J., Wang, S., Tian, S., He, Y., Lou, H., Yang, Z., et al. (2018). Nobiletin inhibits breast cancer via p38 mitogen-activated protein kinase, nuclear transcription factor-κB, and nuclear factor erythroid 2-related factor 2 pathways in MCF-7 cells. Food Nutr Res, 62. https://doi.org/10.29219/fnr.v62.1323
Chen, C., Ono, M., Takeshima, M., & Nakano, S. (2014). Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Research, 34(4), 1785–1792.
Morley, K. L., Ferguson, P. J., & Koropatnick, J. (2007). Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Letters, 251(1), 168–178. https://doi.org/10.1016/j.canlet.2006.11.016
Jiang, Y. P., Guo, H., & Wang, X. B. (2018). Nobiletin (NOB) suppresses autophagic degradation via over-expressing AKT pathway and enhances apoptosis in multidrug-resistant SKOV3/TAX ovarian cancer cells. Biomedicine & Pharmacotherapy, 103, 29–37. https://doi.org/10.1016/j.biopha.2018.03.126
Deveci Ozkan, A., Kaleli, S., Onen, H. I., Sarihan, M., Guney Eskiler, G., Kalayci Yigin, A., et al. (2020). Anti-inflammatory effects of nobiletin on TLR4/TRIF/IRF3 and TLR9/IRF7 signaling pathways in prostate cancer cells. Immunopharmacology and Immunotoxicology, 42(2), 93–100. https://doi.org/10.1080/08923973.2020.1725040
Chen, J., Creed, A., Chen, A. Y., Huang, H., Li, Z., Rankin, G. O., et al. (2014). Nobiletin suppresses cell viability through AKT pathways in PC-3 and DU-145 prostate cancer cells. BMC Pharmacology and Toxicology, 15, 59. https://doi.org/10.1186/2050-6511-15-59
Sun, Y., Han, Y., Song, M., Charoensinphon, N., Zheng, J., Qiu, P., et al. (2019). Inhibitory effects of nobiletin and its major metabolites on lung tumorigenesis. Food & Function, 10(11), 7444–7452. https://doi.org/10.1039/c9fo01966a
Luo, G., Guan, X., & Zhou, L. (2008). Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line A549 in vitro and in vivo. Cancer Biology & Therapy, 7(6), 966–973. https://doi.org/10.4161/cbt.7.6.5967
Lellupitiyage Don, S. S., Robertson, K. L., Lin, H. H., Labriola, C., Harrington, M. E., Taylor, S. R., et al. (2020). Nobiletin affects circadian rhythms and oncogenic characteristics in a cell-dependent manner. PLoS ONE, 15(7), e0236315. https://doi.org/10.1371/journal.pone.0236315
Wang, Y., Solt, L. A., Kojetin, D. J., & Burris, T. P. (2012). Regulation of p53 stability and apoptosis by a ROR agonist. PLoS ONE, 7(4), e34921. https://doi.org/10.1371/journal.pone.0034921
Brożyna, A. A., Kim, T. K., Zabłocka, M., Jóźwicki, W., Yue, J., Tuckey, R. C., et al. (2020). Association among vitamin D, retinoic acid-related orphan receptors, and vitamin D hydroxyderivatives in ovarian cancer. Nutrients, 12(11). https://doi.org/10.3390/nu12113541
Wang, J., Zou, J. X., Xue, X., Cai, D., Zhang, Y., Duan, Z., et al. (2016). ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nature Medicine, 22(5), 488–496. https://doi.org/10.1038/nm.4070
Liu, X., Zawidzka, E. M., Li, H., Lesch, C. A., Dunbar, J., Bousley, D., et al. (2019). RORγ agonists enhance the sustained antitumor activity through intrinsic Tc17 cytotoxicity and Tc1 recruitment. Cancer Immunology Research, 7(7), 1054–1063. https://doi.org/10.1158/2326-6066.Cir-18-0714
Chang, M. R., Dharmarajan, V., Doebelin, C., Garcia-Ordonez, R. D., Novick, S. J., Kuruvilla, D. S., et al. (2016). Synthetic RORγt agonists enhance protective immunity. ACS Chemical Biology, 11(4), 1012–1018. https://doi.org/10.1021/acschembio.5b00899
Mahalingam, D., Wang, J. S., Hamilton, E. P., Sarantopoulos, J., Nemunaitis, J., Weems, G., et al. (2019). Phase 1 open-label, multicenter study of first-in-class RORγ agonist LYC-55716 (Cintirorgon): Safety, tolerability, and preliminary evidence of antitumor activity. Clinical Cancer Research, 25(12), 3508–3516. https://doi.org/10.1158/1078-0432.Ccr-18-3185
Hirota, T., Lee, J. W., St John, P. C., Sawa, M., Iwaisako, K., Noguchi, T., et al. (2012). Identification of small molecule activators of cryptochrome. Science, 337(6098), 1094–1097. https://doi.org/10.1126/science.1223710
Chun, S. K., Chung, S., Kim, H. D., Lee, J. H., Jang, J., Kim, J., et al. (2015). A synthetic cryptochrome inhibitor induces anti-proliferative effects and increases chemosensitivity in human breast cancer cells. Biochemical and Biophysical Research Communications, 467(2), 441–446. https://doi.org/10.1016/j.bbrc.2015.09.103
Fulcher, L. J., & Sapkota, G. P. (2020). Functions and regulation of the serine/threonine protein kinase CK1 family: Moving beyond promiscuity. The Biochemical Journal, 477(23), 4603–4621. https://doi.org/10.1042/bcj20200506
Hirota, T., Lee, J. W., Lewis, W. G., Zhang, E. E., Breton, G., Liu, X., et al. (2010). High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIα as a clock regulatory kinase. PLoS Biology, 8(12), e1000559. https://doi.org/10.1371/journal.pbio.1000559
Janovská, P., Normant, E., Miskin, H., & Bryja, V. (2020). Targeting casein kinase 1 (CK1) in hematological cancers. Int J Mol Sci, 21(23). https://doi.org/10.3390/ijms21239026
Shen, C., Nayak, A., Melendez, R. A., Wynn, D. T., Jackson, J., Lee, E., et al. (2020). Casein kinase 1α as a regulator of Wnt-driven cancer. Int J Mol Sci, 21(16). https://doi.org/10.3390/ijms21165940
Rosenberg, L. H., Lafitte, M., Quereda, V., Grant, W., Chen, W., Bibian, M., et al. (2015). Therapeutic targeting of casein kinase 1δ in breast cancer. Sci Transl Med, 7(318), 318ra202. https://doi.org/10.1126/scitranslmed.aac8773
Bibian, M., Rahaim, R. J., Choi, J. Y., Noguchi, Y., Schürer, S., Chen, W., et al. (2013). Development of highly selective casein kinase 1δ/1ε (CK1δ/ε) inhibitors with potent antiproliferative properties. Bioorganic & Medicinal Chemistry Letters, 23(15), 4374–4380. https://doi.org/10.1016/j.bmcl.2013.05.075
Minzel, W., Venkatachalam, A., Fink, A., Hung, E., Brachya, G., Burstain, I., et al. (2018). Small molecules co-targeting CKIα and the transcriptional kinases CDK7/9 control AML in preclinical models. Cell, 175(1), 171-185.e125. https://doi.org/10.1016/j.cell.2018.07.045
Acknowledgements
We thank the members from the Academy of Integrative Medicine for the helpful discussions. We apologize to the scientists whose work could not be cited in this review due to space limitations.
Funding
This work was supported by the National Science Foundation of China (82172947 to F.H.).
Author information
Authors and Affiliations
Contributions
YW, HDG, and FH conceived the structure of the manuscript and revised the manuscript. YW wrote the manuscript and made the figures. HDG and FH reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Guo, H. & He, F. Circadian disruption: from mouse models to molecular mechanisms and cancer therapeutic targets. Cancer Metastasis Rev 42, 297–322 (2023). https://doi.org/10.1007/s10555-022-10072-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10072-0