Abstract
Purpose
Observational studies and randomized controlled trials (RCTs) have shown an association between vitamin D levels and prostate cancer progression. However, evidence of direct causality is sparse and studies have not examined biological mechanisms, which can provide information on plausibility and strengthen the evidence for causality.
Methods
We used the World Cancer Research Fund International/University of Bristol two-stage framework for mechanistic systematic reviews. In stage one, both text mining of published literature and expert opinion identified testosterone as a plausible biological mechanism. In stage two, we performed a systematic review and meta-analysis to assess the evidence from both human and animal studies examining the effect of vitamin D on testosterone, and testosterone on advanced prostate cancer (diagnostic Gleason score of ≥ 8, development of metastasis) or prostate cancer-specific mortality.
Results
A meta-analysis of ten human RCTs showed evidence of an effect of vitamin D on total testosterone (standardised mean difference (SMD) = 0.133, 95% CI = − 0.003–0.269, I2 = 0.0%, p = 0.056). Five human RCTs showed evidence of an effect of vitamin D on free testosterone (SMD = 0.173, 95% CI = − 0.104–0.450, I2 = 52.4%, p = 0.220). Three human cohort studies of testosterone on advanced prostate cancer or prostate cancer-specific mortality provided inconsistent results. In one study, higher levels of calculated free testosterone were positively associated with advanced prostate cancer or prostate cancer-specific mortality. In contrast, higher levels of dihydrotestosterone were associated with lowering prostate cancer-specific mortality in another study. No animal studies met the study eligibility criteria.
Conclusion
There is some evidence that vitamin D increases levels of total and free testosterone, although the effect of testosterone levels within the normal range on prostate cancer progression is unclear. The role of testosterone as a mechanism between vitamin D and prostate cancer progression remains inconclusive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common cancer in men in the UK with approximately 48,500 newly diagnosed cases and is the second cause of male-related cancer mortality in the UK [1]. Prostate-specific antigen (PSA) screening has led to many men being diagnosed with localised prostate cancer [2]. However, although many men are diagnosed with localised prostate cancer in old age, the majority of these tumours do not progress to become advanced tumours and the use of PSA screening to identify men with localised prostate cancer has been shown to have little effect on prostate cancer-specific mortality when compared to usual care (Incidence rate ratio = 0.96, 0.85–1.08) [3]. In a large randomized controlled trial of men diagnosed with localised prostate cancer who were followed up over a median of 10 years, only around 8% of men were found to have evidence of disease progression over this time period [4]. With many men diagnosed and living with prostate cancer, it is important to identify modifiable exposures that increase men’s risk of prostate cancer progression. The association of vitamin D with cancer progression outcomes, including prostate cancer, has received much attention [5,6,7].
Vitamin D, a fat-soluble vitamin, is essential to the absorption of calcium from the gut into the bloodstream and regulates circulating phosphate levels and bone mineralisation. Vitamin D is available through three sources: sunlight, plant-based and fortified foods (e.g., breakfast cereals), and supplements, and is hydroxylated in the liver and kidneys to produce calcitriol (the active hormone) [8].
Evidence exists of an association between vitamin D on prostate cancer progression and mortality. For example, a meta-analysis of cohort studies with 7808 participants found higher circulating 25(OH)D vitamin D levels to be associated with reduced risk of prostate cancer-specific mortality (Hazard ratio = 0.91, 95% CI = 0.87–0.97, p = 0.002, I2 = 53.4%) [9]. Intervention studies have found that vitamin D supplementation results in a lower number of repeat positive biopsy cores (55% reduction [10]) at a one year follow-up and lower prostate-specific antigen levels [11] at 6–8 weeks follow-up among men with low and intermediate stage prostate cancer.
The strength of evidence for, and plausibility of, an effect of vitamin D on prostate cancer progression (i.e., Gleason scores of ≥ 8, metastasis, prostate cancer-specific mortality) may be improved if studies which examine potential underpinning mechanistic pathways are considered. In the current study, we used the World Cancer Research Fund International/University of Bristol two-stage mechanistic review framework to synthesise evidence from a wide range of different study types, including human and animal studies [12]. Stage one involved identifying a relevant biological mechanism for the vitamin D—prostate cancer progression association using text mining approaches. For stage two, the evidence for the mechanism in relation to both the vitamin D exposure and the prostate cancer progression outcome was systematically reviewed. Further details of the methodology are published elsewhere [12].
Testosterone, a male sex hormone produced by the testis and adrenal glands with a critical role in driving cell division in the prostate gland, was chosen as a potential mechanism from our stage one exploration for two main reasons. First, text mining analyses showed that there was a greater quantity of evidence linking testosterone with both vitamin D and prostate cancer than for other potential mechanisms. Second, there is biological plausibility for testosterone having a causal role in prostate cancer; studies by Huggins and colleagues [13, 14] found that testosterone administered after surgical castration of men with metastatic prostate cancer resulted in increased rates of prostate cancer progression. Androgen deprivation therapy (ADT) is subsequently used clinically to reduce testosterone production in the treatment of prostate cancer. Further information on how we selected testosterone as a mechanism in stage 1 of this review is provided in reference 15.
Stage two of our systematic review of mechanisms aimed to synthesize human and animal studies to investigate whether there is evidence that an association of vitamin D on prostate cancer progression could be via an effect of vitamin D on circulating levels of testosterone within the normal range.
Methods
The protocol of this systematic review can be found elsewhere [15]. We conducted the systematic review using the World Cancer Research Fund International/University of Bristol two-stage mechanistic review framework [12]. We reported this systematic review in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis guidelines [16]. A populated checklist for this review has been provided in Supplementary file 1.
Participants
For the studies linking vitamin D to testosterone, we included those on men only or studies presenting data stratified by sex. For the studies linking testosterone to prostate cancer progression outcomes, we included men with pre-diagnostic testosterone concentrations or men diagnosed with localised prostate cancer and a measurement of testosterone at baseline.
Exposures
Vitamin D
We included any duration, frequency, and dose of vitamin D, including nutrition supplements, for intervention studies examining the vitamin D-testosterone association. There were no restrictions on vitamin D exposures in observational studies.
Testosterone
Eligible human studies for the testosterone-prostate cancer analyses included those which measured total testosterone, free testosterone, bioavailable testosterone, or dihydrotestosterone. Most circulating testosterone is bound to two proteins in the blood—albumin and sex hormone binding globulin (SHBG)—and is measured directly in a blood sample as total testosterone. Free testosterone is a fraction of circulating testosterone (approximately 2%) that is unbound to these two proteins and is measured either directly from a blood sample or can be calculated using values of albumin and SHBG. Bioavailable testosterone is the sum of free testosterone and albumin-bound testosterone. Approximately 10% of testosterone is converted to a hormone dihydrotestosterone by certain tissues of the body, including the prostate gland, and is responsible for the growth of the prostate.
Animal studies which examined endogenous testosterone levels on prostate cancer progression association were eligible.
Outcomes
Outcomes of interest were: (i) total tostestorone, free testosterone, and dihydrotestosterone concentrations for vitamin D-testosterone association studies; and (ii) a diagnostic Gleason score of ≥ 8, development of metastasis, and prostate cancer-specific mortality, for studies of testosterone-prostate cancer progression.
Eligible studies
We included original studies published in peer-reviewed articles. There was no restriction on the publication date of the articles or language. Eligible studies included observational studies (prospective cohorts, nested case–control studies), Mendelian randomization studies, human experimental studies (randomised controlled trials, cross-over studies), and animal studies. To evaluate the testosterone-prostate cancer progression association, we limited observational studies to those with a follow-up of at least 2 years or with a median or mean of 5 years between the measurement of testosterone and a diagnosis of advanced cancer or prostate cancer-specific mortality. As we were interested in the effect of normal variation in endogenous testosterone levels on measures of prostate cancer progression and to avoid the possibility of reverse causation, we excluded studies that examined testosterone treatment effects on prostate cancer progression, in particular the effects of ADT. Both vitamin D and testosterone concentrations vary by age. Therefore, observational studies that did not adjust for age in their analyses or where a large difference in age were observed were excluded from the review.
We excluded cross-sectional and retrospective case-only study designs to avoid reverse causation. We also excluded in vitro and xenograft studies, and animal studies presenting cell line data only, as these designs provide weak evidence on mechanisms operating in humans.
Literature searches
We searched the following electronic bibliographic databases for relevant published articles without year or language restrictions: PubMed (from inception to May 2020); Ovid MEDLINE (1946 to May 2020); Ovid EMBASE (1980 to May 2020); and BIOSIS Citation Index (1969 to May 2020). Two sets of searches were performed: (1) studies that linked circulating vitamin D to circulating testosterone; and (2) studies that linked circulating testosterone to measures of prostate cancer progression (i.e., Gleason score of ≥ 8, development of metastases, prostate cancer-specific mortality). Search strategies included standard controlled vocabulary (MeSH and Emtree), text words, and keywords, and were amended to accommodate the individual requirements of each bibliographic database. An information specialist with experience of conducting systematic reviews was consulted to advise on the search strategies for each database, which are shown in Supplementary file 2.
We searched the reference lists of each included article, relevant systematic review articles, and commentaries and letters found within the electronic searches.
Study selection
All titles and abstracts yielded from each search were initially screened for duplicates based on titles, author names, page numbers, years of publication, and journal names. All titles and abstracts were then screened against the inclusion criteria independently by two of four authors (LAR, VYT, RB, SJL). If an abstract was not available or provided insufficient information to inform a screening decision, the full text article was retrieved. The full text of potentially eligible articles identified from the title and abstract screening was retrieved and assessed against the eligibility criteria. Full text articles were screened by two authors (LAR, SH, SJL) and included if a consensus decision was reached. Disagreements in full text screening were resolved through discussion.
Data extraction
Data on the following characteristics were extracted from each included study: study location, demographics (age, ethnicity), study design, exposure measurement (including type, dose, and duration for vitamin D; serum concentration for total and free testosterone and dihydrotestosterone), length of follow-up, and measures of prostate cancer progression (i.e., Gleason score, metastases, and prostate cancer-specific mortality). Statistical data were extracted including: sample size, effect estimate (mean, standard deviation, median, interquartile range, p value, odds ratio, 95% confidence intervals), and whether studies adjusted for age in their analysis. Data were extracted by one author (LR) and checked for accuracy by another author (SH). Discrepancies were resolved through discussion among the authors.
Risk of bias in individual studies
We performed risk of bias (RoB) assessments on each included study. We used the Risk of Bias 2 (RoB 2) tool [17] to assess human randomised controlled trials. The RoB 2 tool assessed the overall RoB for each study using the following rating: high risk of bias, some concerns, low risk of bias, or no information. For human cohort studies, we used a tool developed for a previous systematic review of mechanistic studies [18] that included domains of assessment from the ROBINS-I tool [19] and questions from the CASP cohort assessment [20]. Each tool evaluated bias due to: confounding, selection of participants, missing data, outcome and exposure measurement, and selective reporting of results. All human cohort studies were considered initially to be at moderate RoB before the assessments were performed and remained at moderate risk unless subsequently found to be at a higher RoB. This is because confounding cannot be fully controlled for within these study designs. We did not perform a risk of bias assessment on Mendelian randomisation studies as there is no risk assessment tool available for these studies at present.
Assessment of reporting bias
We assessed the potential for publication bias using a funnel plot and Egger’s test for the vitamin D- testosterone studies where we had more than 10 studies [21].
Grade assessments
We assessed the certainty of evidence for each association using the Grading of Recommendations Assessment, Development and Evaluation system (GRADE; [22]). RCTs were given an a-priori ranking of high and observational studies a ranking of low certainty of evidence. Rankings were subsequently downgraded based on the following five categories: (1) risk of bias; (2) inconsistency of results; (3) indirectness of evidence; (4) imprecision; and (5) reporting bias. A final certainty of evidence rating, which ranged from high, moderate, low, and very low rating, was given to each study after a consensus was reached among four authors (JPTH, RMM, SJL, LAR).
Statistical analyses
We performed a meta-analysis of sufficiently similar studies using STATA version 15 [23]. We estimated the standardised mean difference (SMD) and standard error for each vitamin D-testosterone association study. We calculated the SMD by calculating the difference in means (MD) (intervention mean minus control mean) at final follow-up and dividing by the average standard deviation (SD) of the exposure (SDe) and control (SDc) groups (i.e., average SD = (SDe2 + SDc2/2)2. We estimated standard deviations where studies presented interquartile ranges [24]. All vitamin D and testosterone concentrations included in the meta-analyses were converted to nanograms per millilitre (ng/ml) if they were not reported as such. We performed random effects and fixed effects meta-analyses using the metan STATA command [25]. The degree of inconsistency across studies was assessed using the I2 statistic [26].
Results
Electronic searches of all four databases identified 14,602 articles. Duplicates (n = 3592) were removed, leaving 11,010 articles for screening of titles and abstracts. Full text articles were retrieved (n = 142) and assessed for eligibility, which resulted in the identification of 16 studies for data extraction and RoB assessments. Hand searching of included study reference lists did not find any additional articles. Figure 1 presents a PRISMA flowchart showing the route to identification of the selected studies via the database searches.
Vitamin D-testosterone studies
Thirteen studies examined the vitamin D-testosterone association. Twelve of these studies were human randomised controlled trials (3 factorial; 9 parallel group) that examined the effects of vitamin D supplementation compared to placebo. The other study was a Mendelian randomisation study [27]. All 13 measured total testosterone (including 1 using a genetic risk score as a proxy measure), five measured free testosterone, and one measured bioavailable testosterone. Two out of the 12 RCTs were judged at high risk of bias. One of these performed a per-protocol analysis only [28] and the other reported higher levels of vitamin D (a greater difference than would be expected by chance) in the intervention group compared to the control groups at baseline [29]. Five studies were judged as having some concerns of bias arising from the randomisation process and deviations from the intended interventions. The remaining five studies had a low risk of bias (Fig. 2). There were no animal studies identified which examined the link between vitamin D and endogenous testosterone concentrations within normal ranges. From the above studies, we obtained ten effect sizes for total testosterone [29,30,31,32,33,34,35], 5 for free testosterone [29, 30, 32,33,34], and one for bioavailable testosterone [30]. Table 1 presents the descriptive characteristics of the vitamin D-testosterone studies.
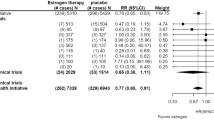
A meta-analysis of the 10 individual study effect sizes for total testosterone found evidence of an effect of vitamin D on total testosterone. Each 1 SD increase in vitamin D was associated with an increased level of total testosterone by 0.133 of an SMD (95% CI = − 0.003–0.269, I2 = 0.0%, p = 0.056) (Fig. 3). There was no evidence of small study effects indicating publication bias (Egger’s test p = 0.535). Table 2 present the effect sizes and standard errors of the vitamin D-testosterone studies.
Whilst the evidence for free testosterone was not strong, the effect was in the same direction and of a similar magnitude. In a meta-analysis of five studies which had assessed this, the increase in the SMD for free testosterone was 0.173 (95% CI = − 0.104–0.450, I2 = 52.4%, p = 0.220) (Fig. 4). There was no evidence of small study effects indicating publication bias (Egger’s test p = 0.405 (Fig. 5).
One RCT [30] found little evidence of an effect of vitamin D supplementation on bioavailable testosterone (SMD = − 0.156, 95% CI = − 0.696–0.384), but the effect estimate for this was in the opposite direction to those for the meta-analysed studies of total testosterone and free testosterone.
We were not able to extract effect sizes related to the effect of vitamin D from two studies of total testosterone and were not able to extract data on free testosterone from one of these studies, as data were presented in a figure only [36, 37]. One of these RCTs [36] found evidence of a decrease in total testosterone and free testosterone following 12 weeks of vitamin D supplementation. The other RCT [37] showed a 41.2% increase in total testosterone following 8 weeks of vitamin D supplementation.
An MR study by Chen et al., [27] created a genetic risk score, using four single nucleotide polymorphisms (see Table 1) previously found to be associated with vitamin D levels, to estimate its effect on total testosterone in 4254 men. The authors found that using the genetic risk score as an instrument a standard deviation increase in 25-hydroxyvitamin D was associated with an increase in total testosterone levels (Beta-coefficient = 0.12, 95% CI = 0.02–0.22).
Testosterone-prostate cancer progression studies
Three human cohort studies reported on the association of total testosterone, free testosterone, and dihydrotestosterone on either prostate cancer-specific mortality alone [38] or in combination with the development of metastasis [39] or a Gleason score of ≥ 8 [40]. All three studies were judged at moderate RoB (Table 3). These three cohort studies could not be meta-analysed due to the significant differences in their reported outcomes. We, therefore, describe their results below and in Table 4. No animal studies were identified which examined endogenous testosterone concentrations on measures of prostate cancer progression.
Kjellman [38] investigated the effects of dihydrotestosterone measured at the time of a prostate cancer diagnosis on prostate cancer-specific mortality. A sample of 65 men with a median age of 65 years were identified from a population-based prostate cancer screening study with a median follow-up of 12.8 (range 1.1–15.3) years. Men with a biopsy-confirmed diagnosis of prostate cancer were included in Kjellman’s study. The authors reported that men with a median dihydrotestosterone value above 0.67 ng/L had a lower mortality rate than those below the median (log rank p = 0.0075).
Gershman [39] examined the effects of pre-diagnostic total and free testosterone and dihydrotestosterone on lethal prostate cancer (defined as development of metastasis or prostate cancer-specific mortality) in men with a mean age of 69 years and a mean follow-up of 12 years. The authors found evidence of an association between total testosterone (HR = 0.95, 95% CI = 0.78–1.16, p = 0.62) or free testosterone (HR = 0.88, 95% CI = 0.60–1.29, p = 0.50) and a reduced risk of lethal prostate cancer.
Pierorazio [40] evaluated the effects of pre-diagnostic total and free testosterone on high-risk prostate cancer (defined as prostate cancer-specific mortality, a PSA level of > 20 ng/mL or a Gleason score of ≥ 8 at diagnosis) in a cohort study of 145 men with a mean age of 52 years and a median follow-up of 22 years. The authors found evidence of an association between calculated free testosterone (ng/dL) and high-risk prostate cancer (OR = 1.61, 95% CI = 1.18–2.20, p = 0.003). There was little evidence of an association between total testosterone (ng/dL) and high-risk prostate cancer (HR = 1.00, 95% CI = 0.998–1.007, p = 0.28).
Grade assessments
We downgraded the certainty of evidence of the effect of vitamin D on total testosterone by two points from high certainty to low certainty due to; the risk of bias in individual studies (two studies were at high risk of bias and five studies had some concerns of bias) (1 point); indirectness of evidence (the studies were from very heterogeneous populations of men including studies in athletes and another in men with chronic heart failure and most were not representative of the target population) (0.5 point); and reporting bias because total testosterone was a primary outcome in only approximately half the number of included studies (0.5 point). We did not downgrade due to imprecision or heterogeneity because there was no evidence of heterogeneity in our meta-analysis and confidence intervals were quite narrow. We downgraded the evidence of the effect of vitamin D on free testosterone by 3 points from high certainty to very low certainty based on the same criteria for the evidence on total testosterone, although with additional points for heterogeneity between the studies included in the meta-analysis (I2 = 52.4%) (1 point) and imprecision (1 point). We downgraded the certainty of the evidence of the effect of testosterone on prostate cancer progression from low to very low certainty due to the imprecision of the results (1 point), heterogeneity between studies (1 point) and publication bias (1 point).
Discussion
We performed a systematic review to investigate whether there was evidence that testosterone concentrations could explain an association of vitamin D with prostate cancer progression. A meta-analysis of 10 RCTs found evidence of a positive association between vitamin D supplementation on total testosterone concentrations. However, we assessed the overall evidence for this association as being of low certainty. We were unable to meta-analyse three studies assessing the association of testosterone with measures of prostate cancer progression. One of the three studies showed an association of pre-diagnostic calculated free testosterone on prostate cancer-specific mortality or advanced prostate cancer (i.e., diagnostic Gleason score of ≥ 8, metastasis). A contradictory finding was observed for dihydrotestosterone which was associated with improved mortality [38]. We assessed the overall certainty of the evidence relating to the association of testosterone with prostate cancer progression as very low.
Our finding of the vitamin D- total testosterone association is supported by a previous published systematic review of 10 RCTs that included 1,061 men [41]. All 10 RCTs were identified in our review, although we excluded one RCT from our review due to strong evidence of a difference in age at baseline between intervention and control groups [42]. We identified 3 additional RCTs [29, 35, 36] which were published since the authors performed their literature searches. In the previous meta-analysis, the authors found little evidence that vitamin D supplementation altered total testosterone levels (mean difference = 0.20, 95% CI = − 0.20–0.60, p = 0.336). The 3 additional studies in our review are likely to have increased the precision of our overall result. The authors used weighted mean differences in their meta-analysis, whilst our review using standardised mean differences. However, there is unlikely to be important differences in the magnitude of effect based on these parameters [43]. Another previous review [44] examined this association in men with and without vitamin D deficiency (i.e., 25 (OH)D below 20 ng/mL). Participants included 9892 men with vitamin D deficiency and 10,675 controls from 18 case-control studies. The authors reported a small association between vitamin D and total testosterone (SMD = − 0.23, 95% CI = − 0.45 to − 0.01; p = 0.04). However, all case–control studies were cross-sectional and were at a higher risk of reverse causation, confounding, and measurement error, and the findings should be interpreted with caution.
Due to the small number of studies assessing the association of testosterone with prostate cancer progression, we were unable to draw any strong conclusions. A systematic review by Claps and colleagues [45] investigated the association between total testosterone and overall mortality (including prostate cancer-specific mortality). In their meta-analysis of four cohort studies [38, 39, 46, 47], the authors found little evidence of an association of total testosterone with overall mortality (HR = 1.03, 95% CI = 0.99–1.08, p = 0.19). Two of these cohort studies were not included our review as one study reported on men treated with ADT [46] and the other study reported on overall survival [47], not prostate cancer-specific survival. It is, therefore, evident that further research would benefit from examining testosterone concentrations in relation to prostate cancer-specific mortality as well as on measures of advanced prostate cancer (i.e., Gleason scores of ≥ 8, development of metastasises).
Limitations
There are several limitations with regards to the included studies and their reported outcomes. Almost half of the vitamin D-testosterone studies were judged as having at least some concerns of risk of bias. Few studies reported on the ethnicity of the participants, although all studies were conducted in countries where the population is predominantly white. We can assume that the findings in these studies are not representative of black men who are at increased risk of prostate cancer [48]. There were differences in the definition of prostate cancer progression, and we were unable to meta-analyse studies assessing associations of testosterone with prostate cancer progression due to the heterogeneity in their reported outcomes. All data included in the meta-analysis were from published peer-reviewed articles. We did not contact subject experts regarding any unpublished or published studies which were not identified from our literature searches.
Implications for future research
We found evidence of an association of increased total testosterone concentrations in men using vitamin D supplementation. However, we found that the overall certainty in the robustness of the finding was low, indicating that further RCTs with total testosterone as a primary outcome with follow-ups of at least 1 year could improve the quality of this evidence. Our review highlights the need for more evidence on the testosterone-prostate cancer progression association. We assessed the certainty of the findings related to this association as very low. Further research could be supported with more cohort studies investigating testosterone as an exposure on well-defined outcomes of prostate cancer progression. Future studies could explore testosterone as a mechanism using large prospective studies which measure vitamin D and testosterone at least 2 years before a diagnosis of prostate cancer. Testosterone could be included in a mediation analysis to assess the effect of vitamin D (exposure) on measures of prostate cancer progression (e.g., PSA or Gleason score as the outcome) through testosterone levels (mediator).
Conclusion
We found evidence of an effect of vitamin D on circulating total testosterone concentrations in men. We did not find strong evidence of an association of testosterone concentrations on prostate cancer progression. Further research is required to establish whether testosterone is a plausible biological mechanism between vitamin D and prostate cancer progression.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
No applicable.
References
Cancer Research UK. Prostate cancer statistics Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Zero. Accessed 4 June 2021
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V et al (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360(13):1320–1328
Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A et al (2018) Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ (Clin Res ed) 362:k3519
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P et al (2016) 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 375(15):1415–1424
Chen L, Davey Smith G, Evans DM, Cox A, Lawlor DA, Donovan J et al (2009) Genetic variants in the vitamin d receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer epidemiol biomarkers prev 18(11):2874–2881
Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM et al (2019) Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: The SUNSHINE randomized clinical trial. JAMA 321(14):1370–1379
Urashima M, Ohdaira H, Akutsu T, Okada S, Yoshida M, Kitajima M et al (2019) Effect of Vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA 321(14):1361–1369
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ (2014) The role of vitamin D in reducing cancer risk and progression. Nat Rev cancer 14(5):342–357
Song ZY, Yao Q, Zhuo Z, Ma Z, Chen G (2018) Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr connect 7(12):R294-r303
Marshall DT, Savage SJ, Garrett-Mayer E, Keane TE, Hollis BW, Horst RL et al (2012) Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocrinol Metab 97(7):2315–2324
Wagner D, Trudel D, Van der Kwast T, Nonn L, Giangreco AA, Li D et al (2013) Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and Ki67 labeling in prostate cancer patients. J Clin Endocrinol Metab 98(4):1498–1507
Lewis SJ, Gardner M, Higgins J, Holly JMP, Gaunt TR, Perks CM et al (2017) Developing the WCRF International/University of Bristol methodology for identifying and carrying out systematic reviews of mechanisms of exposure-cancer associations. Cancer epidemiol biomarkers prev 26(11):1667–1675
Huggins C, Hodges CV (1941) Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J Urol 1:293–297
Huggins CJAS (1941) Studies on prostatic cancer: II. The effect of castration on clinical patients with carcinoma of the prostate. Arch Surg 43:209
Robles LA, Dawe K, Martin RM, Higgins JPT, Lewis SJ (2019) Does testosterone mediate the relationship between vitamin D and prostate cancer? A systematic review and meta-analysis protocol. Syst Rev 8(1):52
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed) 372:n71
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed) 366:l4898
Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M, Perks C et al (2017) Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control 28(6):497–528
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin Res ed) 355:i4919
Programme CAS. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Cohort-Study-Checklist_2018.pdf. Accessed 17 May 2021
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clin Res ed) 343:d4002
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin Res ed) 336(7650):924–926
StataCorp (2017) Stata Statistical Software: release 15. College Station, StataCorp LLC
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14(1):135
Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JA (2008) Metan: fixed-and random-effects meta-analysis. Stand Genomic Sci 8(1):3–28
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Chen C, Zhai H, Cheng J, Weng P, Chen Y, Li Q et al (2019) Causal link between vitamin d and total testosterone in men: a mendelian randomization analysis. J Clin Endocrinol Metab 104(8):3148–3156
Saha S, Goswami R, Ramakrishnan L, Vishnubhatla S, Mahtab S, Kar P et al (2018) Vitamin D and calcium supplementation, skeletal muscle strength and serum testosterone in young healthy adult males: Randomized control trial. Clin Endocrinol (Oxf) 88(2):217–226
Michalczyk MM, Gołaś A, Maszczyk A, Kaczka P, Zając A (2020) Influence of sunlight and oral D(3) supplementation on serum 25(OH)D concentration and exercise performance in elite soccer players. Nutrients. https://doi.org/10.3390/nu12051311
Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B et al (2011) Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res = Horm Stoffwechselforschung = Horm metab 43(3):223–225
Heijboer AC, Oosterwerff M, Schroten NF, Eekhoff EM, Chel VG, de Boer RA et al (2015) Vitamin D supplementation and testosterone concentrations in male human subjects. Clin Endocrinol (Oxf) 83(1):105–110
Jorde R, Grimnes G, Hutchinson MS, Kjaergaard M, Kamycheva E, Svartberg J (2013) Supplementation with vitamin D does not increase serum testosterone levels in healthy males. Horm Metab Res = Horm Stoffwechselforschung = Horm metab 45(9):675–681
Lerchbaum E, Pilz S, Trummer C, Schwetz V, Pachernegg O, Heijboer AC et al (2017) Vitamin D and testosterone in healthy men: a randomized controlled trial. J Clin Endocrinol Metab 102(11):4292–4302
Lerchbaum E, Trummer C, Theiler-Schwetz V, Kollmann M, Wölfler M, Heijboer AC et al (2019) Effects of vitamin D supplementation on androgens in men with low testosterone levels: a randomized controlled trial. Eur J Nutr 58(8):3135–3146
Mielgo-Ayuso J, Calleja-González J, Urdampilleta A, León-Guereño P, Córdova A, Caballero-García A et al (2018) Effects of vitamin d supplementation on haematological values and muscle recovery in elite male traditional rowers. Nutrients. https://doi.org/10.3390/nu10121968
Rockwell MS, Frisard MI, Rankin JW, Zabinsky JS, McMillan RP, You W et al (2020) Effects of seasonal vitamin D3 supplementation on strength, power, and body composition in college swimmers. Int J Sport Nutr Exerc Metab. https://doi.org/10.1123/ijsnem.2019-0250
Scholten SD, Sergeev IN, Song Q, Birger CB (2015) Effects of vitamin D and quercetin, alone and in combination, on cardiorespiratory fitness and muscle function in physically active male adults. Open Access J Sports Med 6:229–239
Kjellman A, Akre O, Norming U, Törnblom M, Gustafsson O (2008) Dihydrotestosterone levels and survival in screening-detected prostate cancer: a 15-yr follow-up study. Eur urol 53(1):106–111
Gershman B, Shui IM, Stampfer M, Platz EA, Gann PH, Sesso HL et al (2014) Prediagnostic circulating sex hormones are not associated with mortality for men with prostate cancer. Eur Urol 65(4):683–689
Pierorazio PM, Ferrucci L, Kettermann A, Longo DL, Metter EJ, Carter HB (2010) Serum testosterone is associated with aggressive prostate cancer in older men: results from the baltimore longitudinal study of aging. BJU Int 105(6):824–829
Hosseini Marnani E, Mollahosseini M, Gheflati A, Ghadiri-Anari A, Nadjarzadeh A (2019) The effect of vitamin D supplementation on the androgenic profile in men: a systematic review and meta-analysis of clinical trials. Andrologia 51(9):e13343
Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J et al (2019) Vitamin D supplementation does not prevent the testosterone decline in males with advanced heart failure: the EVITA trial. Eur J Nutr 58(2):673–680
Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA (2014) Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol 14:30
D’Andrea S, Martorella A, Coccia F, Castellini C, Minaldi E, Totaro M et al (2020) Relationship of vitamin D status with testosterone levels: a systematic review and meta-analysis. Endocrine. https://doi.org/10.1007/s12020-020-02482-3
Claps M, Petrelli F, Caffo O, Amoroso V, Roca E, Mosca A et al (2018) Testosterone levels and prostate cancer prognosis: systematic review and meta-analysis. Clin Genitourin Cancer 16(3):165–75.e2
Roach M, Bae K, Lawton C, Donnelly BJ, Grignon D, Hanks GE et al (2010) Baseline serum testosterone in men treated with androgen deprivation therapy and radiotherapy for localized prostate cancer. Int J Radiat Oncol*Biol*Phys 78(5):1314–1322
Taussky D, Souliéres D, Azoulay L, Yin H, Bahig H, Bahary JP et al (2017) A combination of testosterone and white blood cell count as a predictive factor of overall survival in localized prostate cancer. Target Oncol 12(5):695–701
Tsodikov A, Gulati R, de Carvalho TM, Heijnsdijk EAM, Hunter-Merrill RA, Mariotto AB et al (2017) Is prostate cancer different in black men? Answers from 3 natural history models. Cancer 123(12):2312–2319
Acknowledgments
We would like to thank Sarah Dawson for her help with the database search strategies.
Funding
This review is funded by the World Cancer Research Fund International (Grant No. WCRF 2015/1421). AM, RMM and SJL were supported by a Cancer Research UK (C18281/A29019) programme grant (the Integrative Cancer Epidemiology Programme). JPTH, RMM, SJL, and LAR were supported by the National Institute of Health and Care Research Bristol Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. JPTH was supported by the NIHR Applied Research Collaboration West at University Hospitals Bristol and Weston NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the WCRF, CRUK, MRC, NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
SJL, RMM, and JPTH are co-investigators who were involved in the conception and design of the review, in the interpretation of the results and in deciding the GRADE rating. LAR was involved in all stages of the review. VYT, RB, and SJL were involved in the title and abstract screening. VYT, RB, and AM were involved in the risk of bias assessments. SH and SJL were involved in the full text screening. SH was involved in the meta-analysis. The first draft of the manuscript was written by LAR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare there to be no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Robles, L.A., Harrison, S., Tan, V.Y. et al. Does testosterone mediate the relationship between vitamin D and prostate cancer progression? A systematic review and meta-analysis. Cancer Causes Control 33, 1025–1038 (2022). https://doi.org/10.1007/s10552-022-01591-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-022-01591-w