Abstract
Purpose
Here, we investigated the potential predictive and elucidating efficacy of cell-free DNA (cfDNA) changes on clinical outcomes and biological effects, respectively, after short-term palbociclib and fulvestrant treatment for patients with hormone receptor (HR)-positive and human epidermal growth factor 2 (HER2)-negative advanced or metastatic breast cancer (ABC).
Methods
In this secondary analysis of the Japan Breast Cancer Research Group-M07 (FUTURE) trial, blood cfDNA was obtained before palbociclib treatment and on day 15 of cycle one (28-day cycle). Target enrichment was performed using next-generation sequencing; progression-free survival (PFS) was compared based on cfDNA changes between baseline and day 15 of cycle one after combination therapy.
Results
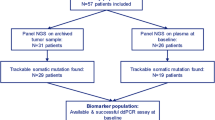
Fifty-six patients (112 paired blood samples) were examined. The median follow-up time was 8.9 months. PIK3CA (30.4%, 17/56), FOXA1 (30.4%, 17/56), and ESR1 (28.6%, 16/56) were most frequently mutated at baseline. The number of mutated genes was significantly decreased on day 15 compared with that at baseline (paired t test: P value = 0.025). No significant difference was observed in PFS (decrease group, 7.9 m vs the others, 9.3 m; log-rank P value = 0.75; hazard ratio, 1.13; 95% confidence interval, 0.53–2.41). Among patients without previous aromatase inhibitor treatment (n = 15), three (20%) had ESR1 mutations after progression to fulvestrant.
Conclusion
No significant association was observed between changes in mutated genes after short-term palbociclib and fulvestrant treatment and disease progression; a significant reduction in cfDNA mutation level was observed on day 15 of cycle one. Clinical meanings of cfDNA should be investigated in the future trials.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to no approval from the Ethics Committee for sharing data.
Abbreviations
- HR:
-
Hormone receptor
- HER2:
-
Human epidermal growth factor 2
- ABC:
-
Advanced or metastatic breast cancer
- PFS:
-
Progression-free survival
- OS :
-
Overall survival
- QOL :
-
Quality of life
- CDK :
-
Cyclin-dependent kinase
- ER :
-
Estrogen receptor
- POETIC :
-
Perioperative endocrine therapy for individualizing care
- IHC :
-
Immunohistochemistry
- AI :
-
Aromatase inhibitor
- cfDNA :
-
Cell-free DNA
- ESMO :
-
European Society for Medical Oncology
- ET :
-
Endocrine therapy
- NGS :
-
Next-generation sequencing
- UMT :
-
Unique molecular tag
- UMI:
-
Unique molecular identifiers
- HR :
-
Hazard ratios
- CI :
-
Confidence intervals
- PR:
-
Progesterone receptor
References
Cardoso F, Senkus E, Costa A et al (2018) 4th ESO-ESMO International Consensus Guidelines for advanced breast cancer (ABC 4)†. Ann Oncol Off J Eur Soc Med Oncol 29:1634–1657. https://doi.org/10.1093/annonc/mdy192
Hortobagyi GN (1998) Treatment of breast cancer. N Engl J Med 11:974–984
Turner NC, Ro J, André F et al (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373:209–219. https://doi.org/10.1056/NEJMoa1505270
Finn RS, Crown JP, Lang I et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16:25–35. https://doi.org/10.1016/S1470-2045(14)71159-3
Finn RS, Martin M, Rugo HS et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925–1936. https://doi.org/10.1056/NEJMoa1607303
Fry DW, Harvey PJ, Keller PR et al (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3:1427–1438
Chu X-J, DePinto W, Bartkovitz D et al (2006) Discovery of [4-Amino-2-(1-methanesulfonylpiperidin-4-ylamino)pyrimidin-5-yl](2,3-difluoro-6- methoxyphenyl)methanone (R547), a potent and selective cyclin-dependent kinase inhibitor with significant in vivo antitumor activity. J Med Chem 49:6549–6560. https://doi.org/10.1021/jm0606138
Jiang P, Lo YMD (2016) The long and short of circulating cell-free DNA and the ins and outs of molecular diagnostics. Trends Genet TIG 32:360–371. https://doi.org/10.1016/j.tig.2016.03.009
Corcoran RB, Chabner BA (2018) Application of cell-free DNA analysis to cancer treatment. N Engl J Med 379:1754–1765. https://doi.org/10.1056/NEJMra1706174
Bettegowda C, Sausen M, Leary RJ et al (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6:224ra24. https://doi.org/10.1126/scitranslmed.3007094
Abbosh C, Birkbak NJ, Wilson GA et al (2017) Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545:446–451. https://doi.org/10.1038/nature22364
Zhou J, Wang C, Lin G et al (2021) Serial circulating tumor DNA in predicting and monitoring the effect of neoadjuvant chemoradiotherapy in patients with rectal cancer: a prospective multicenter study. Clin Cancer Res Off J Am Assoc Cancer Res 27:301–310. https://doi.org/10.1158/1078-0432.CCR-20-2299
Bidard F-C, Hardy-Bessard A-C, Dalenc F et al (2022) Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 23:1367–1377. https://doi.org/10.1016/S1470-2045(22)00555-1
Pantel K, Alix-Panabières C (2019) Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat Rev Clin Oncol 16:409–424. https://doi.org/10.1038/s41571-019-0187-3
Hadad S, Iwamoto T, Jordan L et al (2011) Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat 128:783–794. https://doi.org/10.1007/s10549-011-1612-1
Smith I, Robertson J, Kilburn L et al (2020) Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol 21:1443–1454. https://doi.org/10.1016/S1470-2045(20)30458-7
Iwamoto T, Katagiri T, Niikura N et al (2017) Immunohistochemical Ki67 after short-term hormone therapy identifies low-risk breast cancers as reliably as genomic markers. Oncotarget 8:26122–26128. https://doi.org/10.18632/oncotarget.15385
Hyman DM, Smyth LM, Donoghue MTA et al (2017) AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol 35:2251–2259. https://doi.org/10.1200/JCO.2017.73.0143
https://pins.japic.or.jp/pdf/newPINS/00068468.pdf. Accessed date 15 Aug 2023
Moss J, Zick A, Grinshpun A et al (2020) Circulating breast-derived DNA allows universal detection and monitoring of localized breast cancer. Ann Oncol 31:395–403. https://doi.org/10.1016/j.annonc.2019.11.014
Watanabe K, Niikura N, Kikawa Y et al (2023) Fulvestrant plus palbociclib in advanced or metastatic hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer after fulvestrant monotherapy: Japan Breast Cancer Research Group-M07 (FUTURE trial). Breast Cancer Res Treat. https://doi.org/10.1007/s10549-023-06911-5
Shi Q, Shao K, Jia H et al (2022) Genomic alterations and evolution of cell clusters in metastatic invasive micropapillary carcinoma of the breast. Nat Commun 13:111. https://doi.org/10.1038/s41467-021-27794-4
Kaminska K, Akrap N, Staaf J et al (2021) Distinct mechanisms of resistance to fulvestrant treatment dictate level of ER independence and selective response to CDK inhibitors in metastatic breast cancer. Breast Cancer Res BCR 23:26. https://doi.org/10.1186/s13058-021-01402-1
Fribbens C, O’Leary B, Kilburn L et al (2016) Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 34:2961–2968. https://doi.org/10.1200/JCO.2016.67.3061
Zhang K, Hong R, Xu F et al (2018) Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res 10:2573–2580. https://doi.org/10.2147/CMAR.S173193
Tolaney SM, Toi M, Neven P et al (2022) Clinical significance of PIK3CA and ESR1 mutations in circulating tumor DNA: analysis from the MONARCH 2 study of abemaciclib plus fulvestrant. Clin Cancer Res 28:1500–1506. https://doi.org/10.1158/1078-0432.CCR-21-3276
Spoerke JM, Gendreau S, Walter K et al (2016) Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 7:11579. https://doi.org/10.1038/ncomms11579
O’Leary B, Cutts RJ, Liu Y et al (2018) The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 8:1390–1403. https://doi.org/10.1158/2159-8290.CD-18-0264
Bianchini G, Malorni L, Arpino G et al (2022) Abstract GS3–07: circulating tumor DNA (ctDNA) dynamics in patients with hormone receptor positive (HR+)/HER2 negative (HER2−) advanced breast cancer (aBC) treated in first line with ribociclib (R) and letrozole (L) in the BioItaLEE trial. Cancer Res 82:GS3-07-GS3-07. https://doi.org/10.1158/1538-7445.SABCS21-GS3-07
Acknowledgements
We greatly appreciate all women who participated in this trial. We also thank all investigators and their collaborators for their dedication to this study, Mebics for their data entry assistance, TAKARA bio for genomic analysis and the Japan Breast Cancer Research Group (JBCRG) for their administrative assistance.
Funding
This trial was founded by AstraZeneca K.K.
Author information
Authors and Affiliations
Contributions
Drafting the work: TI; conception or design of the work: TI, NN, KW, TT, YK, NI, HI, NM, and SS; acquisition of data and samples: KK, TO, HT, SO, TO, UT, YY, and MT; Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Conflict of interest
T Iwamoto: research grant from Pfizer; N Niikura: research grant from Chugai, Pfizer, Eisai, Mochida, Daiichi Sankyo and Novartis and honoraria for lectures from Chugai, Eli Lilly, MSD, Daiichi-Sankyo, AstraZeneca and Pfizer; K Watanabe: honoraria for lectures from Chugai, Eli Lilly, Nippon-Kayaku, Kyowa-Kirin, Novartis, Taiho, Eisai, Pfizer, Shionogi, Daiichi-Sankyo and AstraZeneca; Y Kikawa: honoraria for lectures from Eisai, Novartis, Astra Zeneca, Taiho, Pfizer, Daiichi Sankyo, Lilly and Chugai; K Kobayashi: honoraria for lectures from Pfizer, Taiho, Chugai, Astrazeneca, Eli Lilly, Eisai and Novartis; H Tada: research grant from Daiichi Sankyo, Eli Lilly, Kirin, Chugai, Novartis, Taiho; U Toh: research grant from Chugai, Eisai, Taiho, Nippon Kayaku and honoraria for lectures from Pfizer, Kyowa-Kirin, Eli Lilly and Daiichi Sankyo; Y Yamamoto: research grant from Chugai, Kyowa-Kirin, Eisai, Daiichi-Sankyo, Nippon-Kayaku, Taiho, Takeda, Lilly, Pfizer and Novartis, honoraria for lectures from AstraZeneca, Chugai, Kyowa-Kirin, Novartis, Lilly, Pfizer, Daiichi-Sankyo, Nippon-Kayaku, Taiho, Eisai, Takeda, MSD, Sysmex and Exact Science, Advisory Board: AstraZeneca, Chugai, Novartis, MSD, Lilly, Pfizer and Daiichi-Sankyo and Member of the Board of Directors at Japanese Breast Cancer Society and Japan Breast Cancer Research Group; H Ishiguro: research grant from Eisai, Daiichi sankyo, Takeda and Chugai and honoraria for lectures from Eisai, Pfizer, Daiichi sankyo, Chugai and Kyowa Kirin; N Masuda: research grant from Chugai, Eli Lilly, Astra Zeneca, Pfizer, Daiichi-Sankyo, MSD, Eisai, Novartis, Sanofi, Kyowa-Kirin, Nippon-Kayaku and Ono-Pharma, honoraria for lectures from Chugai, Pfizer, Astra Zeneca, Eli Lilly, Daiichi Sankyo and Eisai and Board of Directors at Japanese Breast Cancer Society; S Saji: research grant from Taiho, Eisai, Chugai, Takeda, MSD, Astra Zeneca and Daiichi Sankyo, honoraria for lectures from Chugai, Kyowa Kirin, MSD, Novartis, Eisai, Takeda, Daiichi Sankyo, Eli Lilly, Astra Zeneca, Pfizer, Taiho, Ono and Nipponkayaku, Participation on a Data Safety Monitoring Board or Advisory Board at Chugai/Roche, Astra Zeneca, Eli Lilly, Pfizer, Kyowa Kirin, Daiichi Sankyo and MSD and Executive board member at JBCRG, JBCS, JSMO and BIG. The others have no competing interests.
Ethics approval
This study received an approval from the Ethics Committee of Fukushima Medical University School of Medicine and the respective institution.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
All study participant consent for publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10549_2023_7144_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1 Individual cfDNA mutations at baseline and day 15 of cycle one after combination treatment. Black: mutation positive; Blank: mutation negative. (TIF 146 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwamoto, T., Niikura, N., Watanabe, K. et al. Changes in cell-free DNA after short-term palbociclib and fulvestrant treatment for advanced or metastatic hormone receptor-positive and human epidermal growth factor 2-negative breast cancer. Breast Cancer Res Treat 203, 225–234 (2024). https://doi.org/10.1007/s10549-023-07144-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07144-2