Abstract
Purpose
Neoadjuvant chemotherapy (NAC) for triple-negative breast cancer (TNBC) allows for assessment of tumor pathological response and has survival implications. In 2017, the CREATE-X trial demonstrated survival benefit with adjuvant capecitabine in patients TNBC and residual disease after NAC. We aimed to assess national rates of NAC for cT1–2N0M0 TNBC before and after CREATE-X and examine factors associated with receiving NAC vs adjuvant chemotherapy (AC).
Methods
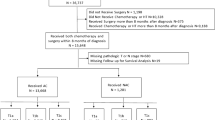
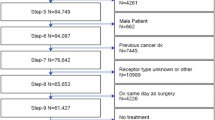
A retrospective cohort study of women with cT1-2N0M0 TNBC diagnosed from 2014 to 2019 in the National Cancer Database (NCDB) was performed. Variables were analyzed via ANOVA, Chi-squared, Fisher Exact tests, and a multivariate linear regression model was created.
Results
55,633 women were included: 26.9% received NAC, 52.4% AC, and 20.7% received no chemotherapy (median ages 53, 59, and 71 years, p < 0.01). NAC utilization significantly increased over time: 19.5% in 2014–15 (n = 3,465 of 17,777), 27.1% in 2016–17 (n = 5,140 of 18,985), and 33.6% in 2018–19 (n = 6,337 of 18,871, p < 0.001). On multivariate analysis, increased NAC was associated with younger age (< 50), non-Hispanic white race/ethnicity, lack of comorbidities, cT2 tumors, care at an academic or integrated-network cancer program, and diagnosis post-2017 (p < 0.05 for all). Patients with government-provided insurance were less likely to receive NAC (p < 0.01). Women who traveled > 60 miles for treatment were more likely to receive NAC (p < 0.01).
Conclusion
From 2014 to 2019, NAC utilization increased for patients with cT1–2N0M0 TNBC. Racial, socioeconomic, and access disparities were observed in who received NAC vs AC and warrants interventions to ensure equitable care.



Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Masuda N, Lee S-J, Ohtani S et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376(22):2147–2159. https://doi.org/10.1056/nejmoa1612645
Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. https://doi.org/10.1056/nejmoa1910549
Mittendorf EA, Zhang H, Barrios CH et al (2020) Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396(10257):1090–1100. https://doi.org/10.1016/S0140-6736(20)31953-X
Loibl S, Schneeweiss A, Huober J et al (2022) Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol 33(11):1149–1158. https://doi.org/10.1016/j.annonc.2022.07.1940
Malorni L, Shetty PB, De Angelis C et al (2012) Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat 136(3):795–804. https://doi.org/10.1007/s10549-012-2315-y
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13(11):674–690. https://doi.org/10.1038/nrclinonc.2016.66
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 13(15):4429–4434. https://doi.org/10.1158/1078-0432.ccr-06-3045
Cho B, Han Y, Lian M et al (2021) Evaluation of racial/ethnic differences in treatment and mortality among women with triple-negative breast cancer. JAMA Oncol 7(7):1016. https://doi.org/10.1001/jamaoncol.2021.1254
Toi M, Lee S-J, Lee ES, et al. A phase III trial of adjuvant capecitabine in breast cancer patients with HER2-negative pathologic residual invasive disease after neoadjuvant chemotherapy (CREATE-X, JBCRG-04). Abstract presented at: San Antonio Breast Cancer Symposium; December, 2015. S1–07.
NCCN Guidelines for Breast Cancer V.3.2017 Meeting Updates. Accessed May 1, 2023.
Telli M, Ward J, Gradishar W. NCCN guidelines updates: Breast cancer. Journal of the National Comprehensive Cancer Network: JNCCN. Accessed May 8, 2023. https://pubmed.ncbi.nlm.nih.gov/31117035/.
Zujewski JA, Rubinstein L (2017) Create-x a role for Capecitabine in early-stage breast cancer: an analysis of available data. Npj Breast Cancer. https://doi.org/10.1038/s41523-017-0029-3
Montemurro F, Nuzzolese I, Ponzone R (2020) Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opin Pharmacother 21(9):1071–1082. https://doi.org/10.1080/14656566.2020.1746273
Matuschek C, Jazmati D, Bölke E et al (2022) Post-neoadjuvant treatment strategies in breast cancer. Cancers 14(5):1246. https://doi.org/10.3390/cancers14051246
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr 30:96–102. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003469
Boughey JC (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer. JAMA 310(14):1455. https://doi.org/10.1001/jama.2013.278932
Le-Petross HT, McCall LM, Hunt KK et al (2018) Axillary ultrasound identifies residual nodal disease after chemotherapy: results from the american college of surgeons oncology group Z1071 trial (Alliance). AJR Am J Roentgenol 210(3):669–676. https://doi.org/10.2214/AJR.17.18295
Boughey JC, Ballman KV, Le-Petross HT et al (2016) Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg 263(4):802–807. https://doi.org/10.1097/SLA.0000000000001375
Caudle AS, Yang WT, Krishnamurthy S et al (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34(10):1072–1078. https://doi.org/10.1200/JCO.2015.64.0094
Korde LA, Somerfield MR, Carey LA et al (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39(13):1485–1505. https://doi.org/10.1200/JCO.20.03399
Huo X, Li J, Zhao F et al (2021) The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 21(1):78. https://doi.org/10.1186/s12885-021-07791-y
Fitzpatrick A, Tutt A (2019) Controversial issues in the neoadjuvant treatment of triple-negative breast cancer. Ther Adv Med Oncol. 11:1758835919882581. https://doi.org/10.1177/1758835919882581
Bilimoria KY, Stewart AK, Winchester DP, Ko CY (2008) the national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15(3):683–690. https://doi.org/10.1245/s10434-007-9747-3
About the National Cancer Database. American College of Surgeons. Accessed May 3, 2023. https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/about/
Martinez EO, Jorns JM, Kong AL et al (2022) Primary breast neuroendocrine tumors: an analysis of the national cancer database. Ann Surg Oncol 29(10):6339–6346. https://doi.org/10.1245/s10434-022-12123-w
Mills MN, Yang GQ, Oliver DE et al (2018) Histologic heterogeneity of triple negative breast cancer: a national cancer centre database analysis. Eur J Cancer 98:48–58. https://doi.org/10.1016/j.ejca.2018.04.011
Ong CT, Campbell BM, Thomas SM et al (2018) Metaplastic breast cancer treatment and outcomes in 2500 patients: a retrospective analysis of a national oncology database. Ann Surg Oncol 25(8):2249–2260. https://doi.org/10.1245/s10434-018-6533-3
Deyo R (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619. https://doi.org/10.1016/0895-4356(92)90133-8
Duchesneau ED, An SJ, Strassle PD et al (2022) Sociodemographic and clinical predictors of neoadjuvant chemotherapy in CT1-T2/n0 Her2-amplified breast cancer. Ann Surg Oncol 29(5):3051–3061. https://doi.org/10.1245/s10434-021-11260-y
Zhang H, Barner JC, Moczygemba LR, Rascati KL, Park C, Kodali D (2022) Neoadjuvant chemotherapy use trends among older women with breast cancer: 2010–2017. Breast Cancer Res Treat 193(3):695–705. https://doi.org/10.1007/s10549-022-06604-5
Puig CA, Hoskin TL, Day CN, Habermann EB, Boughey JC (2016) National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a national cancer data base study. Ann Surg Oncol 24(5):1242–1250. https://doi.org/10.1245/s10434-016-5733-y
Lorentzen T, Heidemann LN, Möller S, Bille C (2022) Impact of neoadjuvant chemotherapy on surgical complications in breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol 48(1):44–52. https://doi.org/10.1016/j.ejso.2021.09.007
Brown L, Carr MJ, Sam C et al (2023) Tolerance and outcomes of neoadjuvant chemotherapy in geriatric breast cancer patients. J Surg Res 283:329–335. https://doi.org/10.1016/j.jss.2022.10.092
Curigliano G, Burstein HJ, Winer EP et al (2017) De-escalating and escalating treatments for early-stage breast cancer The St Gallen international expert consensus conference on the primary therapy of early breast cancer. Annals of Oncology 28(8):1700–1712. https://doi.org/10.1093/annonc/mdx308
Hershman DL, Till C, Wright JD et al (2016) Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest oncology group clinical trials. J Clin Oncol 34(25):3014–3022. https://doi.org/10.1200/jco.2015.66.2346
Johnson-Arbor K, Dubey R. Doxorubicin. In: Statpearls. StatPearls Publishing; 2023.
Ogino M, Tadi P. Cyclophosphamide. In: Statpearls. StatPearls Publishing; 2022.
Thompson AM, Moulder-Thompson SL (2012) Neoadjuvant treatment of breast cancer. Ann Oncol 23:x231–x236. https://doi.org/10.1093/annonc/mds324
Newman LA, Kaljee LM (2017) Health disparities and triple-negative breast cancer in african american women: a review. JAMA Surg 152(5):485–493. https://doi.org/10.1001/jamasurg.2017.0005
Shavers VL, Brown ML (2002) Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 94(5):334–357. https://doi.org/10.1093/jnci/94.5.334
Hershman D, McBride R, Jacobson JS et al (2005) Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol 23(27):6639–6646. https://doi.org/10.1200/JCO.2005.12.633
Reyes SA, King TA, Fei K, Franco R, Bickell NA (2016) Factors affecting the completion of adjuvant chemotherapy in early-stage breast cancer. Ann Surg Oncol 23(5):1537–1542. https://doi.org/10.1245/s10434-015-5039-5
Zipkin RJ, Schaefer A, Wang C et al (2022) Rural-Urban differences in breast cancer surgical delays in medicare beneficiaries. Ann Surg Oncol 29(9):5759–5769. https://doi.org/10.1245/s10434-022-11834-4
Connors SK, Goodman MS, Myckatyn T, Margenthaler J, Gehlert S (2016) Breast reconstruction after mastectomy at a comprehensive cancer center. Springerplus. 5(1):955. https://doi.org/10.1186/s40064-016-2375-2
Black DM, Jiang J, Kuerer HM, Buchholz TA, Smith BD (2014) Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg 149(8):788–796. https://doi.org/10.1001/jamasurg.2014.23
Cortina CS, Bergom CR, Kijack J, Thorgerson AA, Huang CS, Kong AL (2021) Postmastectomy breast reconstruction in women aged 70 and older: An analysis of the national cancer database (NCDB). Surgery 170(1):30–38. https://doi.org/10.1016/j.surg.2021.03.033
Hershman D, Weinberg M, Rosner Z et al (2003) Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst 95(20):1545–1548. https://doi.org/10.1093/jnci/djg073
Hershman DL, Unger JM, Barlow WE et al (2009) Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest oncology studies S8814/S8897. J Clin Oncol 27(13):2157–2162. https://doi.org/10.1200/JCO.2008.19.1163
Bickell NA, Wang JJ, Oluwole S et al (2006) Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol 24(9):1357–1362. https://doi.org/10.1200/JCO.2005.04.5799
Lipscomb J, Gillespie TW, Goodman M et al (2012) Black-white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat 133(1):285–296. https://doi.org/10.1007/s10549-011-1916-1
Daly B, Olopade OI (2015) A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 65(3):221–238. https://doi.org/10.3322/caac.21271
Ward E, Jemal A, Cokkinides V et al (2004) Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 54(2):78–93. https://doi.org/10.3322/canjclin.54.2.78
Magai C, Consedine NS, Adjei BA, Hershman D, Neugut A (2008) Psychosocial influences on suboptimal adjuvant breast cancer treatment adherence among African American women: implications for education and intervention. Health Educ Behav 35(6):835–854. https://doi.org/10.1177/1090198107303281
Chen JY, Diamant AL, Thind A, Maly RC (2008) Determinants of breast cancer knowledge among newly diagnosed, low-income, medically underserved women with breast cancer. Cancer 112(5):1153–1161. https://doi.org/10.1002/cncr.23262
Freedman RA, Virgo KS, He Y et al (2011) The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer 117(1):180–189. https://doi.org/10.1002/cncr.25542
Mujumdar V, Butler TR, Shalowitz DI (2021) A qualitative study on the impact of long-distance travel for gynecologic cancer care. Gynecol Oncol Rep. 38:100868. https://doi.org/10.1016/j.gore.2021.100868
Wolfe MK, McDonald NC, Holmes GM (2020) Transportation barriers to health care in the United States: findings from the national health interview survey, 1997–2017. Am J Public Health 110(6):815–822. https://doi.org/10.2105/AJPH.2020.305579
Wheeler SB, Spencer JC, Pinheiro LC, Carey LA, Olshan AF, Reeder-Hayes KE (2018) Financial impact of breast cancer in black versus white women. J Clin Oncol 36(17):1695–1701. https://doi.org/10.1200/JCO.2017.77.6310
McDermott AM, Wall DM, Waters PS et al (2013) Surgeon and breast unit volume-outcome relationships in breast cancer surgery and treatment. Ann Surg 258(5):808–814. https://doi.org/10.1097/SLA.0b013e3182a66eb0
Kong AL, Pezzin LE, Nattinger AB (2015) Identifying patterns of breast cancer care provided at high-volume hospitals: a classification and regression tree analysis. Breast Cancer Res Treat 153(3):689–698. https://doi.org/10.1007/s10549-015-3561-6
Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY (2009) Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the national cancer data base. J Clin Oncol 27(25):4177–4181. https://doi.org/10.1200/JCO.2008.21.7018
Tutt ANJ, Garber JE, Kaufman B et al (2021) Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 384(25):2394–2405. https://doi.org/10.1056/NEJMoa2105215
von Minckwitz G, Huang CS, Mano MS et al (2019) Trastuzumab Emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380(7):617–628. https://doi.org/10.1056/NEJMoa1814017
Esserman L (2016) The I-SPY approach to drug development. Clin Adv Hematol Oncol 14(10):782–784
Campbell JI, Yau C, Krass P et al (2017) Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat 165(1):181–191. https://doi.org/10.1007/s10549-017-4303-8
Funding
CSC is supported by the National Institutes of Health (NIH) under Award Number 1K08CA276706-01A1 (PI: Cortina). The content of this manuscript is soley the responsiblity of the authors and does not neccessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant disclosures to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rogers, C., Cobb, A.N., Lloren, J.I.C. et al. National trends in neoadjuvant chemotherapy utilization in patients with early-stage node-negative triple-negative breast cancer: the impact of the CREATE-X trial. Breast Cancer Res Treat 203, 317–328 (2024). https://doi.org/10.1007/s10549-023-07114-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07114-8