Abstract
Purpose
Multiple treatment options exist for patients with metastatic breast cancer (MBC). However, limited information is available on the impact of prior treatment duration and class on survival outcome for novel therapies, such as cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+ HER2−) MBC.
Methods
This study used a nationwide, de-identified electronic health record-derived database to identify women with HR+ HER2− MBC who received at least one CDK 4/6i between 2011 and 2020. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated for the association between prior duration and class of cancer treatment (both early-stage and metastatic) and prior CDK 4/6i survival as well as for those with multiple CDK 4/6i.
Results
Of 5363 patients, the median survival from first CDK 4/6 inhibitor administration was 3.3 years. When compared to patients with no prior treatments, patients with < 1 year of prior treatment duration had a 30% increased hazard of death (HR, 1.30; 95% CI 1.15–1.46), those with 1 to < 3 years a 68% increased hazard of death (HR 1.68; 95% CI 1.49–1.88), and those with 3 or more years a 55% increased hazard of death (HR 1.55; 95% CI 1.36, 1.76). Patients who received prior therapy (endocrine or chemotherapy) before their CDK 4/6i had worse outcomes than those who received no prior therapy. Similar results were seen when comparing patients in the metastatic setting alone. Finally, patients who received a different CDK 4/6i after their first saw a lower hazard of death compared to patients who received subsequent endocrine or chemotherapy after their first CDK 4/6i.
Conclusion
Prior treatment duration and class are associated with a decreased overall survival after CDK 4/6 inhibitor administration. This highlights the importance for clinicians to consider prior treatment and duration in treatment decision-making and for trialists to stratify by these factors when randomizing patients or reporting results of future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For patients with metastatic breast cancer (MBC), there are a lack of consensus on the optimal treatment sequencing. Within the NCCN guidelines, over 40 regimens are available across subtypes. In hormone receptor-positive, human epidermal growth factor 2-negative (HR+ HER2−) MBC, the most common subtype accounting for 50–60% of cases, more than 20 treatment regimens are listed [1, 2]. This heterogeneity results in a large number of available options for treatment sequencing. Our previous study found that among 6639 sequences of MBC therapy, there were 3718 rare sequences and only 44% of patients received a sequence that 11 other patients had received [3]. Thus, patients presenting with similar characteristics can receive different guideline-based treatment sequences, which may lead to differing outcomes.
The PALOMA, MONARCH, and MONALEESA trials established cyclin-dependent kinase (CDK) 4/6 inhibitors, Palbociclib, Abemaciclib, and Ribociclib, respectively, as standard of care for patients with HR+ HER2− MBC based on observed improved progression-free survival and overall survival benefits ranging from 9.5 to 20.5 months. [4,5,6] However, these trials reported minimal information regarding the class or duration of treatments received prior to the treatment of interest. The PALOMA-3 trial evaluated Fulvestrant and Palbociclib reporting the frequency of previous treatments as 1, 2, or ≥ 3 with no discussion regarding previous treatment duration [4]. The MONARCH-3 trial of Abemaciclib only reported yes or no to previous chemotherapy or endocrine therapy [7]. Prior studies have shown that early-line treatments influence the response to later-line treatments [8,9,10,11]. Therefore, a better understanding of the impact of prior time on treatment, prior treatment class, and prior use of CDK 4/6 inhibitors is needed to apply these treatments to patients. This study leverages real-world data to evaluate the association between prior treatments (both early stage and metastatic) duration and class (e.g., endocrine therapy, chemotherapy, CDK 4/6 inhibitor) and overall survival using both traditional modeling and a visualization technique. [3]
Methods
Study design and sample
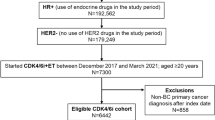
This retrospective cohort study used the nationwide, electronic health record (EHR)-derived Flatiron Health de-identified database to include women diagnosed with metastatic breast cancer between 2011 and 2020. Flatiron Health is a longitudinal database composed of de-identified patient-level structured and unstructured data curated from approximately 280 US oncology care sites (~ 800 sites of care), including community cancer practices and academic medical centers [12]. The study population included women with HR+ HER2− MBC from Flatiron Health’s MBC database. Inclusion criteria included biomarker information to identify the HR and HER2 statuses prior to their CDK 4/6 inhibitor initiation and patients must have received a CDK 4/6 inhibitor (Palbociclib, Abemaciclib, or Ribociclib) during their treatment course with either no previous treatment, previous endocrine therapy, or previous chemotherapy. The cohort excluded patients who were male, aged less than 18 years old, had missing cancer subtype or treatment, or had suspected incorrect treatment (e.g., receiving docetaxel and paclitaxel at the same time). This study was approved by the University of Alabama at Birmingham Internal Review Board prior to study conduct and included a waiver of informed consent.
Variables
Outcome and overall survival
The primary outcome for this study is overall survival (OS), defined as time from initiation of the first CDK 4/6 inhibitor to death as a result of any cause. Death was recorded in the Flatiron Health database by aggregating structured and unstructured EHR records, Social Security Death Index, and obituaries. [13]
Patient characteristics
Patients’ age was determined by the date of their primary breast cancer diagnosis. Age was then categorized as less than 45, 45 to 54, 55 to 64, 65 to 74, and 75 or older. Similarly, patients’ race or ethnicity was categorized as White, Black, Other, or not documented. Other race included Hispanic or Latino, Asian, American Indian, Alaskan Native, and Pacific Islanders; these races were combined due to small sample sizes in the dataset.
Clinical characteristics
Patients' sites of metastasis were categorized as visceral, bone, or lymph node only. Patients with at least one visceral site were considered visceral, those without visceral sites but with at least one bone site were considered bone, and those with only lymph node sites were considered lymph node only. Cancer subtype was determined through hormone receptor (HR, estrogen receptor and progesterone receptor) and human epidermal growth factor receptor 2 (HER2) biomarker tests. Patients were considered positive for a biomarker if any tests were positive.
Treatment characterization
Prior treatments were defined as any cancer treatment (early or advanced stage) received prior to a CDK 4/6 inhibitor. Treatments were identified using generic drug names recorded as either administered or ordered alongside treatment start and end dates. Cancer treatment duration was defined as the total duration of all cancer treatments—including targeted, endocrine therapy, and/or chemotherapy—received after the patients’ initial cancer diagnosis and prior to the initiation of the first recorded CDK 4/6 inhibitor. Similarly, prior treatment duration in the metastatic setting was defined as the total duration of cancer treatments from the patients’ metastatic diagnosis date to the first recorded CDK 4/6 inhibitor. Prior treatment durations were categorized as 0 years (i.e., no prior treatment), < 1 year, 1 to < 3 years, and 3 or more years. The type of cancer treatments prior to CDK 4/6 inhibitor initiation were categorized as chemotherapy (taken with or without endocrine and targeted therapies), endocrine therapy (with or without targeted therapies; no chemotherapy), or no prior treatment (frontline CDK 4/6 inhibitor in the metastatic setting). To understand if multiple CDK 4/6 inhibitors are associated with improved survival, a subset of patients with at least one treatment after the CDK4/6 inhibitors was identified. In this subset, patients were categorized as receiving either a second CDK 4/6 inhibitor or another treatment (e.g., chemotherapy (taken with or without therapy) or endocrine therapy without chemotherapy (targeted therapies allowed)).
Statistical analysis
Descriptive analyses included medians and interquartile ranges (IQRs) for continuous variables or frequencies and percentages for categorical variables. The median OS from the initiation of the first CDK 4/6 inhibitor was calculated using the Kaplan–Meier estimator. The associations between OS and total treatment duration and class prior to CDK 4/6 inhibitor initiation were estimated using hazard ratios (HRs) and 95% confidence intervals (CI) from Cox proportional hazard models. Additional analysis included only prior treatment duration and class in the metastatic setting (i.e., treatments after metastatic diagnosis date and prior to the first CDK 4/6 inhibitor). The models were adjusted for age at diagnosis, race and ethnicity, site of metastasis, and metastatic diagnosis year.
For the analysis comparing OS for patients who received a second CDK 4/6 inhibitor vs. another treatment, two HR estimates were computed: (1) an estimate from a Cox proportional hazard model with a time-dependent indicator variable for second CDK 4/6 and (2) an estimate from a prescription time-distribution matched analysis. The model for the first HR estimate was adjusted for age at diagnosis, race and ethnicity, site of metastasis, prior treatment duration (years), first CDK 4/6 inhibitor type, first CDK 4/6 inhibitor duration, and year of first CDK 4/6 inhibitor. For the matched analysis, propensity scores were computed using a non-linear, non-parametric random forest ensemble modeling those with a second CDK 4/6 inhibitor vs. those with another treatment (not CDK4/6 inhibitor) with the aforementioned control variables. Next, radius matching on the propensity score (radius = 0.001) was conducted in addition to selecting each match by being alive at the time of the respective 2nd CDK 4/6 and having a number of therapy lines after first CDK 4/6 equal or greater to the number of therapy lines from the respective first to second CDK 4/6. Then an HR estimate from the matched data was computed. HRs from 1-to-1, 2-to-1, and 3-to-1 matching runs were computed. Analyses were performed using R, version 4.0.5 and SAS© software, version 9.4 (SAS Institute, Cary, NC).
Visualization
A graphic displaying a random sample of 150 patients’ treatments and their duration was created and displayed using a novel visualization approach that was developed by our team as seen in a prior publication [3]. The software has been updated and made to be more customizable. To summarize, in this visualization paradigm, patients are represented on the y-axis with treatment time on the x-axis. A color-coded treatment bar represents each treatment class within the patient’s course. CDK 4/6 inhibitors are shown as dark purple, endocrine therapies are shown in teal, chemotherapy in green, and other targeted therapies in orange. Treatment gaps are represented as white space. A Kaplan–Meier curve was overlaid as a function of time from CDK 4/6 initiation (referred to here as time zero) to death or censoring. To assess for using CDK4/6 inhibitors beyond progression, a visualization image was created displaying the second CDK 4/6 inhibitor displayed in light purple. Visualization graphics were created using R, version 4.0.5.
Results
Sample demographics
A total of 5391 women diagnosed with HR+ HER2− MBC were eligible for inclusion. The demographic and clinical characteristics of the study participants are shown in Table 1. Patients were most commonly aged 55–64 (29%), White (69%), and had visceral metastasis (70%). In the adjuvant and metastatic setting, 33% had no prior treatment, 37% received endocrine therapy alone, and 30% chemotherapy alone or chemotherapy with endocrine. Of those who received CDK 4/6 inhibitors as first line, Palbociclib was the most commonly prescribed (84%). In the metastatic setting, no prior treatment (49%) was most common prior to a CDK 4/6 inhibitor, followed by hormone therapy alone (34%) and chemotherapy alone or chemotherapy in combination with hormone therapy (17%). Of the 635 (12%) patients receiving multiple CDK4/6 inhibitors, the most common order was Palbociclib followed by Abemaciclib (54%).
Overall survival
The median OS was 4.3 years for patients with no therapy prior to a CDK 4/6 inhibitor, but 3.3 years when including patients with any prior treatments. After adjusting for age at diagnosis, race and ethnicity, site of metastasis, and metastatic diagnosis year for patients in the adjuvant and metastatic setting, compared to patients who have not received any treatment, patients with a prior treatment duration of < 1 year had a 30% increased hazard of death (HR, 1.30; 95% CI 1.15, 1.46), those with a prior treatment duration of 1 to < 3 years had a 68% increased hazard of death (HR 1.68; 95% CI 1.49, 1.88), and those with a prior treatment duration of 3 or more years had a 54% increased hazard of death (HR 1.54; 95% CI 1.36, 1.76; Table 2). For patients in the metastatic setting only, compared to patients who received no prior treatment, those who received a prior treatment duration of < 1 year had a 25% increased hazard of death (HR, 1.25; 95% CI 1.13, 1.39), those with a prior treatment duration of 1 to < 3 years had a 39% increased hazard of death (HR 1.39; 95% CI 1.21, 1.61), and those with a prior treatment duration of 3 or more years had a 9% increased hazard of death (HR 1.09; 95% CI 0.87, 1.36). Similar results were seen when analyzing the class of treatment received prior to CDK 4/6 inhibitor administration. In the adjuvant and metastatic setting, patients receiving prior endocrine therapy had a 29% increased hazard of death (HR, 1.29; 95% CI 1.16, 1.44), while patients receiving prior chemotherapy had a 72% increased hazard of death (HR, 1.72; 95% CI 1.54, 1.93) when compared with patients who did not receive a prior treatment. In the metastatic setting, patients receiving prior endocrine therapy had a 23% increased hazard of death (HR, 1.23; 95% CI 1.11, 1.36), while patients receiving prior chemotherapy had a 57% increased hazard of death (HR, 1.57; 95% CI 1.39, 1.78) when compared with patients who did not receive a prior treatment.
The data contained n = 595 patients who received a second CDK 4/6 inhibitor and n = 2926 who received treatments other than a second CDK 4/6 inhibitors in the subsequent line. Table 3 shows HRs comparing OS for patients who received a second CDK 4/6 inhibitor vs. endocrine, chemotherapy, or other non-CDK4/6-targeted therapies. For the time-dependent analysis, patients who received another CDK 4/6 inhibitor after their first CDK 4/6 inhibitor had a 17% decreased hazard of death when compared with patients who received a single CDK 4/6 inhibitor (HR, 0.83; 95% CI 0.71, 0.96). Additional matched analysis revealed that patients who received another CDK 4/6 inhibitor after their first CDK 4/6 inhibitor had a 21% decreased hazard of death when compared with patients who received only a single CDK 4/6 inhibitor (HR, 0.79; 95% CI 0.65, 0.95). Similar results were seen when 1-to-1 and 1-to-2 matching was analyzed (Supplemental Table 1).
Treatment sequence visualization
Patients who received endocrine (shown in teal) or chemotherapy (shown in green) prior to CDK 4/6 inhibitor initiation had lower survival compared with those who received CDK 4/6 inhibitors earlier in their treatment course (Fig. 1). Patients near the top of the image have longer treatments prior to first CDK 4/6 inhibitor but show shorter post-CDK 4/6 inhibitor survival compared with those at the bottom of the figure with fewer treatments prior to first CDK 4/6 inhibitor. The figure also displays that patients near the bottom of the image had a similar total duration of any treatment to the patients near the top of the image. Figure 2 displays patients who received multiple CDK 4/6 inhibitors throughout their treatment course with the first CDK 4/6 inhibitor being time 0. The initial CDK 4/6 inhibitor is shown in dark purple with the subsequent CDK 4/6 inhibitors shown in lighter purple. Patients with longer first-line CDK 4/6 inhibitors appear to have longer survival and a better response when compared to patients with later-line CDK 4/6 inhibitors.
Treatment course of patients diagnosed with metastatic breast cancer who received a CDK 4/6 inhibitor. Definitions: CDK 4/6 inhibitor, cyclin-dependent kinase 4/6; HER2, human epidermal growth factor receptor 2. Random sample of 150 patients from our cohort in 2015. The x-axis includes time in years with the patients first CDK 4/6 inhibitor at time zero. The y-axis includes individual patients. The image is sorted according to overall survival from CDK 4/6 inhibitor initiation. Patients higher on the x-axis have lower survival post-CDK 4/6 inhibitor initiation than those at the bottom. Patients who received endocrine (teal) or chemotherapy (shown in green) prior to CDK 4/6 inhibitor initiation had lower survival compared with those who received CDK 4/6 inhibitors earlier in their treatment course. (Color figure online)
Treatment course of patients diagnosed with metastatic breast cancer who received multiple CDK 4/6 inhibitors. Definition: CDK 4/6 inhibitor, cyclin-dependent kinase 4/6; HER2, human epidermal growth factor receptor 2. Random sample of 150 patients from our cohort. The x-axis includes time in years with the patients first CDK 4/6 inhibitor at time zero. The y-axis includes individual patients. The image is sorted according to overall survival from the first CDK 4/6 inhibitor. Patients higher on the x-axis have lower survival post-CDK 4/6 inhibitor initiation than those at the bottom. Patients with longer first-line CDK 4/6 inhibitors appear to have longer survival and a better response when compared to patients with later-line CDK 4/6 inhibitors
Discussion
This study demonstrates the importance of prior treatment duration and class to the initiation of a CDK 4/6 inhibitors in women with HR+ HER2– MBC. Patients with longer cancer treatment durations prior to their first CDK 4/6 inhibitor had an increased hazard of death after their CDK 4/6 inhibitor initiation. This finding was particularly noteworthy in patients that received prior chemotherapy. A study evaluating patients receiving Palbociclib and Letrozole demonstrated that patients without prior cancer treatments who received this regimen had a two-fold PFS benefit compared to those with prior endocrine therapy or chemotherapy [14]. Similarly, an analysis of patients with MBC receiving paclitaxel, where patients experienced an increased hazard of death of 71% with one line of treatment before paclitaxel, a 30% increase with two prior treatments, and a 123% increase with three or more prior treatments compared to those with no prior treatments before paclitaxel [15]. Together these studies emphasize the importance of considering both duration and type of prior therapy when interpreting clinical trials results. These findings have implications for trial design and reporting for second- and later-line regimens, promoting the need for statistical analysis that takes heterogeneity of prior therapies into account. Furthermore, this has practical applications for physicians who need to convey prognostic information to patients and must be able to identify how individual patients in clinic are similar or different to the study population.
Another key finding of this study is the survival benefits observed for patients who switch CDK 4/6 inhibitors compared to those who receive subsequent endocrine, chemotherapy, and non-CDK4/6-targeted therapies. Our study finding of a 21% reduced hazard of death supports prior early findings regarding patients receiving multiple CDK4/6 inhibitors. Wander et al. found that patients who progressed to Abemaciclib after receiving Palbociclib had similar efficacy to the MONARCH-1 trial with a median progression-free survival of 5.3 months and a median overall survival on Abemaciclib of 17.2 months. [16, 17] This study was limited by a small sample size (n = 87) and only analyzed the relationship between switching from Palbociclib to Abemaciclib. In contrast, our study evaluated all combinations of CDK4/6 inhibitors in a substantially larger sample (n = 635). While these studies suggest benefit of continued CDK4/6 inhibition, further randomized studies are needed to definitively quantify the magnitude of benefit for second CDK4/6 inhibitor compared to other treatment strategies.
Given the challenge in capturing these higher dimensional data for different treatment sequences and their metadata, we employed our visualization algorithm to help create a topological representation of such heterogeneity [15, 18]. The features of the resultant treatment sequence graph can inform us about sequence frequency, median survival, overall average prior lines, and performance of any specific agent on disease behavior and outcome within the large sequence landscape of breast cancer. For example, by centering the zero time point on CDK4/6 agents, we can see the diverse outcome for our real-world patients. In part, this heterogeneity can be explained by impact of prior and subsequent treatments causing selective pressure with evolutionary expansion of resistant clones, such as CCNE1/2, RB1, and ERBB2 in case of CDK4/6 resistance [19]. Another feature of the graph is the inflection points of survival line, which would roughly describe variability of median survival across grouped sequences. For example, in Figure 1, we see an inflection point separating sequences that contain chemotherapy in the upper part from those that utilize further endocrine therapy below. These features attest to the power of our visualization solution and its adaptability and suitability for describing rather complex yet very important real-world outcomes of therapeutic interventions in breast cancer. Incorporating more higher dimensional data into our visualization tool, such as genomic drivers, intrinsic subtypes, and metastatic burden, can add further features and significant insight into the treatment landscape. The approach of leveraging real-world databases to evaluate regimens after clinical trials are completed can also be applied within cancer types where the paradigm includes sequential therapies. [20, 21]
There are important limitations to consider in this analysis. We were unable to account for comorbidities in our current analysis as current EHR International Classification of Diseases (ICD) codes are not sufficient in identifying comorbidities [22]. This may have overestimated the relationships seen on prior treatment duration and class and post-CDK 4/6 inhibitor survival. We were also unable to account for biological factors such as genetic tumor alterations which could have influenced OS if mutations developed shortened the post-CDK 4/6 inhibitor survival. We also did not focus on a specific CDK 4/6 inhibitor; therefore, we are unable to state whether these results are the same across each type. Similarly, when analyzing patients who switch CDK 4/6 inhibitors we did not differentiate between the order of CDK 4/6 inhibitors; therefore, further research is needed to determine if there is an optimal sequence for patients receiving multiple CDK 4/6 inhibitors. Considering the heterogeneity of MBC treatments, residual confounding may remain as we were unable to account for clinician-specific decision-making and all patient-specific characteristics that may play a role in treatment decisions.
Conclusion
This study found that both duration and type of prior treatment before receipt of CDK 4/6 inhibitors impact survival after receipt of cancer therapy, highlighting the importance of treatment sequencing when interpreting survival outcomes. As additional treatment options are added to the MBC treatment milieu, it is crucial that treatment history in patient cohorts under study be analyzed and integrated in considered in their development to aid in integrating them with other treatment options.
Data availability
Data that support the findings of this study have been originated by Flatiron Health, Inc. These de-identified data may be made available upon request and are subject to a license agreement with Flatiron Health; interested researchers should contact < DataAccess@flatiron.com > to determine licensing terms.
Abbreviations
- MBC:
-
Metastatic breast cancer
- HR+ :
-
Hormone receptor-positive
- HER2−:
-
Human epidermal growth factor 2-negative
- CDK:
-
Cyclin-dependent kinase
- OS:
-
Overall survival
- IQR:
-
Interquartile range
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- PFS:
-
Progression-free survival
- EHR:
-
Electronic health record
- ICD:
-
International classification of diseases
References
National Comprehensive Cancer Network: Invasive breast cancer. (https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf).
Al-Thoubaity FK (2020) Molecular classification of breast cancer: a retrospective cohort study. Ann Med Surg (Lond) 49:44–48. https://doi.org/10.1016/j.amsu.2019.11.021
Rocque GB, Kandhare PG, Williams CP et al (2020) Visualization of sequential treatments in metastatic breast cancer. JCO Clin Cancer Inform 4:1–8. https://doi.org/10.1200/cci.18.00095
Cristofanilli M, Turner NC, Bondarenko I et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439. https://doi.org/10.1016/s1470-2045(15)00613-0
Hui R, de Boer R, Lim E, Yeo B, Lynch J (2021) CDK4/6 inhibitor plus endocrine therapy for hormone receptor-positive, HER2-negative metastatic breast cancer: the new standard of care. Asia Pac J Clin Oncol 17(S1):3–14. https://doi.org/10.1111/ajco.13555
Slamon DJ, Neven P, Chia S et al (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36(24):2465–2472. https://doi.org/10.1200/JCO.2018.78.9909
Goetz MP, Toi M, Campone M et al (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35(32):3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
Bonotto M, Gerratana L, Iacono D et al (2015) Treatment of metastatic breast cancer in a real-world scenario: is progression-free survival with first line predictive of benefit from second and later lines? Oncologist 20(7):719–24. https://doi.org/10.1634/theoncologist.2015-0002
Park IH, Lee KS, Ro J (2015) Effects of second and subsequent lines of chemotherapy for metastatic breast cancer. Clin Breast Cancer 15(1):e55-62. https://doi.org/10.1016/j.clbc.2014.09.001
Yamamura J, Kamigaki S, Tsujie M et al (2019) Response to first-line recurrence treatment influences survival in hormone receptor-positive, her2-negative breast cancer: a multicenter study. In Vivo 33(1):281–287. https://doi.org/10.21873/invivo.11473
Jacquet E, Lardy-Cléaud A, Pistilli B et al (2018) Endocrine therapy or chemotherapy as first-line therapy in hormone receptor-positive HER2-negative metastatic breast cancer patients. Eur J Cancer 95:93–101. https://doi.org/10.1016/j.ejca.2018.03.013
Birnbaum B, Nussbaum NC, Seidl-Rathkopf K, et al. 2020 Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. http://arxiv.org/a2001.09765
Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM (2021) Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res 56(6):1281–1287. https://doi.org/10.1111/1475-6773.13669
Brufsky A, Mitra D, Davis KL et al (2019) Treatment patterns and outcomes associated with palbociclib plus letrozole for postmenopausal women with HR(+)/HER2(−) advanced breast cancer enrolled in an expanded access program. Clin Breast Cancer 19(5):317-325.e4. https://doi.org/10.1016/j.clbc.2019.04.005
Rocque GB, Gilbert A, Williams CP et al (2020) Prior treatment time affects survival outcomes in metastatic breast cancer. JCO Clinical Cancer Informatics 4:500–513. https://doi.org/10.1200/cci.20.00008
Dickler M, Tolaney S, Rugo H (2017) MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2– metastatic breast cancer. Clin Cancer Res 23:5218–5224 ((In English))
Wander SA, Han HS, Zangardi ML et al (2021) Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: a multicenter experience. J Natl Compr Canc Netw 1:8. https://doi.org/10.6004/jnccn.2020.7662
Bukkuri A, Andor N, Darcy IK (2021) Applications of topological data analysis in oncology. Front Artif Intell 4:38. https://doi.org/10.3389/frai.2021.659037
Wander SA, Cohen O, Gong X et al (2020) The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov 10(8):1174–1193. https://doi.org/10.1158/2159-8290.Cd-19-1390
Klonoff DC (2020) The expanding role of real-world evidence trials in health care decision making. J Diabetes Sci Technol 14(1):174–179. https://doi.org/10.1177/1932296819832653
Llewellyn-Bennett R, Edwards D, Roberts N, Hainsworth AH, Bulbulia R, Bowman L (2018) Post-trial follow-up methodology in large randomised controlled trials: a systematic review. Trials 19(1):298. https://doi.org/10.1186/s13063-018-2653-0
Parrinello CM, Seidl-Rathkopf KN, Bourla AB, Nussbaum NC, Carson KR, Abernethy AP (2018) Comparison of structured versus abstracted comorbidities using oncology EHR data from cancer patients in the flatiron health network. Value Health 21:S14. https://doi.org/10.1016/j.jval.2018.04.083
Acknowledgements
We would like to acknowledge Gerald McGwin, PhD for his assistance with this project.
Funding
This work was funded by Pfizer 5552703. They had no role in the design, analysis, interpretation, or writing of this manuscript.
Author information
Authors and Affiliations
Contributions
GR, AA, and JF contributed to study concept/design, GR contributed to provision of study material, JF and AA contributed to data collection/assembly, JF, AA, TG, and GR contributed to data analysis and interpretation. All authors contributed to manuscript writing and final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
GR receives research funding from Genentech, Pfizer, and Carevive and consulting fees for Pfizer, Gilead, and Flatiron.
Ethical approval
This study was approved by the University of Alabama at Birmingham IRB-300006051.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franks, J., Caston, N.E., Elkhanany, A. et al. Effect of prior treatments on post-CDK 4/6 inhibitor survival in hormone receptor-positive breast cancer. Breast Cancer Res Treat 197, 673–681 (2023). https://doi.org/10.1007/s10549-022-06823-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06823-w