Abstract
Purpose
To assess whether crofelemer would prevent chemotherapy-induced diarrhea (CID) diarrhea in patients with HER2-positive, any-stage breast cancer receiving trastuzumab (H), pertuzumab (P), and a taxane (T; docetaxel or paclitaxel), with/without carboplatin (C; always combined with docetaxel rather than paclitaxel).
Methods
Patients scheduled to receive ≥ 3 consecutive TCHP/THP cycles were randomized to crofelemer 125 mg orally twice daily during chemotherapy cycles 1 and 2 or no scheduled prophylactic medication (control). All received standard breakthrough antidiarrheal medication (BTAD) as needed. The primary endpoint was incidence of any-grade CID for ≥ 2 consecutive days. Secondary endpoints were incidence of all-grade and grade 3/4 CID by cycle/stratum; time to onset and duration of CID; stool consistency; use of BTAD; and quality of life (Functional Assessment of Chronic Illness Therapy for Patients With Diarrhea [FACIT-D] score).
Results
Fifty-one patients were randomized to crofelemer (n = 26) or control (n = 25). There was no statistically significant difference between arms for the primary endpoint; however, incidence of grade ≥ 2 CID was reduced with crofelemer vs control (19.2% vs 24.0% in cycle 1; 8.0% vs 39.1%, in cycle 2). Patients receiving crofelemer were 1.8 times more likely to see their diarrhea resolved and had less frequent watery diarrhea.
Conclusion
Despite the choice of primary endpoint being insensitive, crofelemer reduced the incidence and severity of CID in patients with HER2-positive breast cancer receiving P-based therapy. These data are supportive of further testing of crofelemer in CID.
Trial registration
Clinicaltrials.gov, NCT02910219, prospectively registered September 21, 2016.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced diarrhea (CID) is debilitating, with a detrimental impact on quality of life [1, 2]. It occurs in ≤ ~ 80% of patients with breast cancer (BC) receiving trastuzumab and pertuzumab (HP; F. Hoffmann-La Roche Ltd, Basel, Switzerland/Genentech, Inc., South San Francisco, CA, USA), plus a taxane (T) [3, 4]. This reaches grade 3 in ~ 8–12% [5,6,7,8]. Various antidiarrheal agents are available for symptom management [2]; however, none target the underlying mechanism. This is predominantly secretory diarrhea from excess chloride ion and fluid secretion in the intestinal lumen through activation of apical cystic fibrosis transmembrane conductance regulator (CFTR) or calcium-activated chloride channels (CaCC) [9]. CID in HP-containing regimens may be caused by EGFR downregulation and/or blockade, which leads to excess chloride secretion and secretory diarrhea through reversal of the acute inhibitory effect of epidermal growth factor on chloride secretion [10,11,12]. CFTR/CaCC activation by H/P has not been described; however, EGFR inhibition-related CFTR/CaCC-mediated chloride ion secretion [12,13,14,15] would apply to H and P, both of which are associated with diarrhea [5]. Enterocyte apoptosis, impaired regeneration, and the chloride ion-mediated secretory mechanism of diarrhea that occurs with EFGR inhibitors may explain why onset with EGFR inhibitors is not immediate and may worsen over time. This is especially relevant when HP is added to agents that are directly toxic to gastrointestinal cells.
Crofelemer (Napo Pharmaceuticals, Inc., San Francisco, CA, USA) is a novel oral botanical antisecretory, antidiarrheal drug purified from Croton lechleri tree sap [16]. Crofelemer regulates luminal chloride efflux and fluid secretion through the use-dependent inhibition of CFTR and CaCC chloride ion channels in the apical membrane of the intestinal mucosa [17] and is FDA-approved for adult patients with HIV with non-infectious diarrhea receiving antiretroviral therapy [18]. Due to its large molecular size and polarity, crofelemer acts mainly in the lumen and, thus, is typically well tolerated [17, 19,20,21]. Only a negligible amount is systemically absorbed following oral dosing in humans in the fasted or fed state [22]. Since crofelemer is the only known selective and specific use-dependent inhibitory modulator of CFTR and CaCC [17], the HALT-D study (NCT02910219) evaluated prophylaxis of diarrhea with crofelemer in patients with BC receiving HP-containing regimens. However, diarrhea remains a challenging endpoint for clinical trials as it is difficult to measure [23] (Version 4.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events [NCI-CTCAE] merely defines diarrhea as a disorder characterized by frequent and watery bowel movements [24]). Recall bias, time to onset, frequency of bowel movements, frequency of watery stools, diarrhea incidence, diarrhea duration, repeated measures, clustering of events, and clinical impact of diarrhea are characteristics that may interfere with overall evaluation.
In HALT-D, we hypothesized that crofelemer would reduce diarrhea in patients with HER2-positive BC receiving HP and a taxane with or without carboplatin (THP/TCHP; the taxane being paclitaxel or docetaxel; where C was always combined with docetaxel rather than paclitaxel) in the neoadjuvant, adjuvant, or metastatic settings. When HALT-D was planned, there was, and continues to be, no established gold standard endpoint for assessing diarrhea in clinical trials. An expert group published recommendations in 2004, but acknowledged that standard practices for assessment and management are needed [25]. The Bristol Stool Form Scale (BSFS) [26, 27] provides one assessment method, but there is inherent subjectivity in classification, and disparities in endpoints and regulatory guidance that require further investigation in large clinical trials [23].
In the absence of a gold standard endpoint, we selected the incidence of any-grade diarrhea for ≥ 2 consecutive days. Here, we present the primary analysis of HALT-D.
Methods
Oversight
HALT-D was conducted according to Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided written informed consent. Protocol approval was obtained from the Georgetown University Institutional Review Board. Safety data were reviewed semi-annually by an independent Data and Safety Monitoring Committee. Crofelemer was provided by Napo Pharmaceuticals, Inc.
Patients
As previously described [28], eligible patients were ≥ 18 years with any-stage HER2-positive BC, scheduled to receive ≥ 3 consecutive THP/TCHP cycles, had an Eastern Cooperative Oncology Group performance status 0–2, and adequate organ function. Patients with irritable bowel syndrome, colitis, recent antibiotic use, active systemic infection, ostomy, prior total colectomy, fecal incontinence, abdominal radiation, and major abdominal or pelvic surgery within the past 6 months or without recovery of bowel function were excluded.
Trial design and procedures
The study schema and schedule of events are presented in Fig. 1 and Table 1.
Study schema. BID twice daily, C, carboplatin; H trastuzumab, P pertuzumab, T taxane. Reprinted from Clinical Breast Cancer, 17, Gao JJ, Tan M, Pohlmann PR, and Swain SM, HALT-D: A Phase II Evaluation of Crofelemer for the Prevention and Prophylaxis of Diarrhea in Patients With Breast Cancer on Pertuzumab-Based Regimens, 76–78, Copyright (2016), with permission from Elsevier
Patients were observed on-study for three cycles. Chemotherapy/HER2-targeted therapy doses were paclitaxel 80 mg/m2 intravenously (IV) every week (q1w) or docetaxel 75 mg/m2 IV q3w; C area under the concentration–time curve 6 IV q3w; H 8 mg/kg IV loading dose, 6 mg/kg IV maintenance doses q3w; and P 840 mg IV loading, 420 mg IV maintenance q3w.
Patients were randomized 1:1 to crofelemer 125 mg delayed release tablets orally twice a day (BID, or no scheduled prophylactic medication in the control (observation) group during cycles 1 and 2 of chemotherapy/HER2-targeted therapy. Randomization was performed by statisticians in the Georgetown Lombardi Comprehensive Cancer Center Biostatistics and Bioinformatics Shared Resource (Washington, DC, USA) who also generated the randomization table and held the key. Stratification was by chemotherapy regimen (HP with paclitaxel, HP with docetaxel, or docetaxel with CHP). The first crofelemer dose was administered 30–60 min prior to the first chemotherapy/HER2-targeted therapy cycle. All other doses were taken at home. At the beginning of cycles 1 and 2, patients randomized to crofelemer received a diary to record administration date and time during each cycle. This was collected at the end of chemotherapy/HER2-targeted therapy cycles 1 and 2. There was no crofelemer administration during cycle 3 (empiric to use two cycles).
At the start of treatment, all patients received recommendations for standard-of-care breakthrough antidiarrheal medication (BTAD) as needed, with no scheduled antidiarrheal prophylactic medication. Patients also received instructions to record BTAD use in a diary: drug name, dose, and administration date/time during all cycles. The diary was collected at the end of cycles 1, 2, and 3.
Baseline daily number of bowel movements was documented before the start of treatment. Investigators considered all clinical data at each follow-up visit and prospectively documented diarrhea events per NCI-CTCAE v4.0. For patient-reported outcomes (PROs), by Day 1 of cycle 1 all patients received a BSFS illustrated form and bowel movement diary. Patients were instructed to record the date, time, and BSFS stool consistency of each movement during the three cycles. Watery diarrhea was defined as BSFS 6–7; non-watery, as 1–5 [26, 27], for both investigator-assessed outcomes and PROs. The probability of watery diarrhea was calculated by repeated logistic regression for each cycle. Diaries were collected by the study team at the end of each cycle (Table 1).
All patients filled out quality-of-life questionnaires based on Functional Assessment of Chronic Illness Therapy for Patients With Diarrhea (FACIT-D) scores on the first day of each cycle, as well as at the end of study participation.
Statistics
The primary endpoint (incidence of any-grade diarrhea for ≥ 2 consecutive days) was assessed by NCI-CTCAE v4.0. Secondary endpoints included incidence of all-grade and grade 3–4 CID by cycle and by stratum; time to onset and duration of CID; stool consistency; frequency of BTAD use; adverse events by stratum; and FACIT-D total and diarrhea subset scores. If the primary endpoint was not met statistically, the secondary endpoints and overall incidence of all-grade diarrhea in both arms were still evaluated for clinical benefit. Patients were observed for adverse events during three cycles.
Fisher’s exact test was used for comparing binary and categorical variables, and summary statistics and the Wilcoxon test were used for ordinal grade/scale variables. The trial was designed to detect a 40% absolute decrease in incidence of CID (60% to 20%), with a two-sided significance level of 0.10. To analyze the time to onset of diarrhea (event), Log-rank tests were performed. Repeated measures logistic regression with computation by generalized estimating equations (SAS Proc GENMOD) was used to assess the overall probability of having watery diarrhea in each treatment cycle. Recurrent survival model of Prentice–Williams–Peterson for times to resolution of diarrhea was used to analyze duration of diarrhea within each cycle between arms, considering multiple bouts of diarrhea with different durations for one patient within a cycle. The likelihood ratio test (LRT) with an ordinal regression model was used to determine whether there was an interaction between crofelemer effect and chemotherapy regimen. Medians and percentage reductions in median value of watery diarrhea episodes per week were calculated comparing crofelemer versus control. The Wilcoxon rank sum test was conducted for two independent groups and the Wilcoxon signed rank test was conducted for paired data. Safety data are descriptive.
Anticipating a 10% withdrawal rate, enrollment of 52 patients was planned. Statistical analyses were performed using R 3.6.2 (R Foundation, Vienna, Austria) and SAS (SAS Institute, Cary, NC, USA).
Results
Fifty-three patients were enrolled between 02/21/2017 and 08/25/2020 to crofelemer (n = 27) or control (n = 26). One withdrew consent prior to starting and another soon after receiving first dose of chemotherapy (no data collected). These patients were excluded from analysis; 26 and 25, respectively, were analyzed. A further 46 were pre-screened and excluded for reasons such as not meeting eligibility criteria, declining treatment, having already started treatment, and provider decision.
Early treatment discontinuation occurred in six cases: complications of diarrhea (n = 1, control group), chemotherapy regimen changed during study participation for causes other than diarrhea (n = 4), and non-compliance with trial procedures (n = 1).
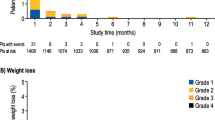
Demographics were well balanced between the two arms (Table 2). Rescue medications included diphenoxylate, diphenoxylate hydrochloride/atropine, diphenoxylate hydrochloride/atropine and loperamide, loperamide, and loperamide and octreotide acetate. Diarrhea was overall more frequent in cycle 1. The primary endpoint was similar between arms. During cycle 1, 68.0% and 69.6% of patients had diarrhea for ≥ 2 consecutive days in the crofelemer and control arms. During cycle 2, the numbers were 65.2% and 72.2% for the control arm (not statistically different: p = 0.742; Fig. 2A).
Several secondary endpoints favored crofelemer (Table 3). Furthermore, in cycle 2, no patients in the crofelemer arm experienced grade 3–4 diarrhea (vs 17.3% in the control arm). Patients in the crofelemer arm experienced significantly less grade ≥ 2 CID than the control arm during cycle 2, based on PROs and investigator reporting (Table 4).
Watery diarrhea of any grade occurred less frequently in the crofelemer arm than in the control arm for cycle 1; the smaller difference between the groups was not statistically significant for cycle 2 (Table 3).
Use of rescue medication for treatment of emerging CID, time to onset, and duration of diarrhea after each cycle of chemotherapy/HER2-targeted therapy were not different between crofelemer and observation at cycle 1 or cycle 2 (data not shown), neither were FACIT-D scores (Online resource 1).
The probability of resolution of CID was evaluated by considering cases with ≥ 1 bout of diarrhea within the same chemotherapy cycle. There were 17/31 patients who had diarrhea in cycle 1 with recurring events (≥ 2 bouts; resolved and occurred again) during cycle 1. The hazard ratio (HR) for CID resolution in cycle 1 was 1.03. There were 19/27 patients who had diarrhea in cycle 2 with recurring diarrhea during cycle 2. The HR for CID resolution in cycle 2 was 1.804, meaning CID in patients in the crofelemer arm was ~ 1.8 times more likely to resolve than in the control arm during cycle 2 (Table 3).
Online resource 2 shows medians and percentage reductions in watery bowel movements from the control arm per week in cycles 1, 2, and 3. Patients in the crofelemer arm received crofelemer only in cycles 1 and 2 (standard of care was given in cycle 3 to all). During cycles 1 and 2, watery bowel movements were less frequent in the crofelemer arm compared with control; however, results were not statistically significant. When comparing cycle 3 with cycle 2 in the crofelemer arm only, medians suggested that patients had more diarrhea during cycle 3; again, results were not statistically significant.
Since randomization was stratified by chemotherapy regimen, potential interactions between crofelemer and regimen (docetaxel- vs paclitaxel-based) could be assessed. CID frequency in cycles 1 and 2 varied according to chemotherapy regimen in patients who received crofelemer (Fig. 2B and C). THP with paclitaxel was more often associated with no diarrhea than the docetaxel-containing regimens (THP or TCHP) but this was not statistically significant at cycles 1 or 2. LRTs of ordinal regression models showed no significant interaction between crofelemer usage and chemotherapy regimen during cycle 2 (LRT = 3.243, degrees of freedom = 1, p = 0.072).
Frequency of the most common non-diarrhea adverse events was similar in both arms (Online resource 3). During cycles 1 and 2, the most frequent were fatigue (n = 10 patients in the crofelemer arm and 9 in the control arm), nausea (n = 10 and 9), anemia (n = 5 and 2), anorexia (n = 4 and 3), mucositis oral (n = 4 and 3), and constipation (n = 4 and 2).
In the crofelemer arm, only one patient experienced a serious adverse event: grade 4 neutropenia attributed to chemotherapy (not crofelemer-related). In the control arm, seven patients experienced serious adverse events: port-a-cath infection, obstruction gastric, cellulitis, hypoglycemia, dehydration, neutropenia, hyperglycemia, hyperkalemia, acidosis, confusion, fatigue, urinary tract infection, chest pain—cardiac, atrial flutter, and fever (all single events and unrelated to treatment; one patient experienced eight events and one patient experienced two). The gastrointestinal obstruction occurred during screening and was unrelated to study procedures. The urinary tract infection occurred in the setting of persistent diarrhea, ultimately leading to intensive care unit admission and treatment discontinuation. The patient with diabetic ketoacidosis was admitted due to poor compliance with insulin treatments and had a complicated hospital course with severe acidosis, changes to glucose and potassium levels, dehydration, and chemotherapy-related neutropenia. After a period of treatment in the intensive care unit, the patient made a complete recovery and resumed treatment.
Discussion
In the HALT-D study of crofelemer for the prevention of CID in patients with HER2-positive BC receiving PH and a taxane, there was no significant difference in the number of patients experiencing ≥ 2 consecutive days of diarrhea between the crofelemer and control arms. It is probable that the selection of this primary endpoint was not the correct choice to adequately determine efficacy of decreasing diarrhea. In the CONTROL study of neratinib-related diarrhea, the primary endpoint was NCI-CTCAE grade ≥ 3 diarrhea. Most patients in the current study experienced any-grade CID for at least 7 days in the 3-week cycle, and many experienced multiple daily episodes (or higher grades) of diarrhea for several days. This makes the selected primary endpoint of ≥ 2 consecutive days insensitive and of limited use in differentiating crofelemer’s effect from that of control. Conversely, there were a number of secondary endpoints that suggested crofelemer benefit. In both treatment cycles, there was a clinically meaningful difference between the crofelemer and control arms in terms of maximum within-cycle ordinal NCI-CTCAE grade diarrhea, which was statistically significant in cycle 2 based on both investigator assessment and PROs. The odds of having watery diarrhea during cycle 1 was 23% lower in the crofelemer arm. Patients in the crofelemer arm were 1.8 times more likely to see their diarrhea resolve than patients in the control arm in cycle 2.
Use of BTAD was permitted on an as-needed basis; there were no significant differences reported between the arms. Pharmacogenomic studies indicate that specific ABCB1 genotype variations have an impact in the plasma concentrations of loperamide [29] and opioids in general [30], which could explain why some patients try to maintain loperamide use, while others may disregard it for lack of efficacy. Similarly, FACIT-D scores did not demonstrate significant differences between treatment groups, which may have been related to the study duration. Patients may not have had adequate exposure to crofelemer to assess potential impact on their quality-of-life scores.
Cancer treatment-related diarrhea management has been “reactive” and few “prophylactic/proactive” approaches have been evaluated. CONTROL [31] required neratinib dose escalation with loperamide to achieve the therapeutic neratinib dose. Patients received rescue budesonide and colestipol, plus dietary recommendations, due to loperamide prophylaxis’ inadequacy in controlling neratinib-induced diarrhea. One arm showed improved tolerability when the neratinib dose was escalated during the first 15 days of therapy. Furthermore, the dose escalation schema of neratinib exposes the patient to sub-therapeutic doses and hence inadequate tumor suppression and/or resistance (to neratinib and potentially other similar agents). Hence, a mechanistically appropriate drug was evaluated in HALT-D to ensure that the loading doses of H and P could be administered, followed by maintenance doses.
THP with paclitaxel and crofelemer was more often associated with no diarrhea than the docetaxel-containing regimens and crofelemer (THP/TCHP). Other studies have shown that diarrhea is less frequent with paclitaxel [32, 33]; however, small numbers in HALT-D may preclude definitive conclusions regarding an interaction and further study would be needed.
The limitations of HALT-D include the selected primary endpoint for this population and treatment regimen. There is a lack of uniformity and agreement on how to assess CID; clinical practice guidelines continue to provide recommendations for treatment only [25, 34]. For that reason, HALT-D evaluated a number of measures of CID, and a decision was made to select the incidence of any-grade diarrhea for ≥ 2 consecutive days, assessed by NCI-CTCAE v4.0, as the primary endpoint. Several other measures were assessed with the intent to provide a comprehensive evaluation of, and to enable discussion of, potential CID endpoints for clinical trials. Another limitation was the short and late exposure to crofelemer. Crofelemer was only administered during the first 2/3 cycles of chemotherapy/HER2-targeted therapy. In terms of timing, the first dose of crofelemer was administered orally at the infusion center, 30 min prior to the first chemotherapy/HER2-targeted therapy dose. This may have been too late to better counteract the effects of IV chemotherapy and anti-HER2 therapies on luminal ion channels, as well as to provide the desired protection to the bowel. In addition, loading doses of P and H were administered at cycle 1, per standard of care.
The strength of HALT-D is it being the first of its kind, to our knowledge, to evaluate the prophylactic use of a novel antidiarrheal drug for preventing or mitigating CID in BC (activated charcoal and budesonide have been shown to possibly mitigate irinotecan-induced diarrhea and reduce loperamide use in small colorectal cancer studies [35, 36]; and a review published in 2019 after HALT-D began highlighted minimal success of prophylaxis with agents available at the time [13]). Since currently used antimotility drugs (loperamide, diphenoxylate/atropine) do not target the CID mechanism, HALT-D evaluated a new paradigm for CID management with crofelemer (a potential targeted therapy). In addition to the new approach for prophylaxis of CID, this is the first study to our knowledge that incorporated PROs to assess incidence and severity of loose/watery stools as a continuous variable in patients with BC. Although HALT-D did not meet its primary endpoint for the reasons outlined above, it still has clinical relevance because the data add to literature on how to evaluate diarrhea in a clinical trial setting. Diarrhea is an important and debilitating side effect of HER2-targeted therapies, and HALT-D helps to better understand how to assess diarrhea interventions in future. HALT-D also represents real-world evidence wherein the patient manages their CID in between cycles of treatments. Finally, data collected by patients themselves were comprehensive in terms of recording and providing the information about their experience during treatment. This allowed extensive collection of prospective daily information for the entire duration of the study, as well as objective analyses of multiple CID endpoints as described.
Future directions include the ongoing phase III, double-blind, placebo-controlled real-world evidence OnTARGET study (NCT04538625), which is evaluating crofelemer for the prophylaxis of diarrhea in adult patients with solid tumors receiving targeted therapy agents with or without chemotherapy. The OnTARGET endpoint integrates various characteristics of targeted therapy-associated diarrhea, including time to onset, duration, and resolution of diarrhea, and the possibility that the diarrhea may be cyclical or intermittent in patients receiving cycles of chemotherapy. It is not a binary endpoint that can be easily reached with highly diarrheagenic treatment regimens. OnTARGET is larger than HALT-D, has longer follow-up, and takes into consideration several elements of diarrhea that HALT-D’s primary endpoint was not able to.
Conclusion
The HALT-D study demonstrated that crofelemer reduces the incidence and severity of grade ≥ 2 CID associated with treatments containing PH and a taxane, especially during the second cycle of TCHP or THP treatment. Furthermore, a significantly larger number of patients in the crofelemer arm versus the control arm either had no loose/watery stools or < 3 loose/watery stools (i.e., grade 1 CID) during the second cycle of chemotherapy/HER2-targeted therapy. Patients in the crofelemer arm were also more likely to see their diarrhea resolve compared with those patients receiving only standard-of-care antidiarrheal medications. The findings of the HALT-D study support further testing of crofelemer in CID.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to Protected Health Information regulations, but are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Adverse event
- BC:
-
Breast cancer
- BID:
-
Twice a day
- BSFS:
-
Bristol Stool Form Scale
- BTAD:
-
Breakthrough antidiarrheal medication
- C:
-
Carboplatin
- CaCC:
-
Calcium-activated chloride channels
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- CI:
-
Confidence interval
- CID:
-
Chemotherapy-induced diarrhea
- FACIT-D:
-
Functional Assessment of Chronic Illness Therapy for Patients with Diarrhea
- FACT-G:
-
Functional Assessment of Cancer Therapy—General
- H:
-
Trastuzumab
- HR:
-
Hazard ratio
- IV:
-
Intravenously
- LRTs:
-
Likelihood ratio tests
- NCI-CTCAE:
-
National Cancer Institute’s Common Terminology Criteria for Adverse Events
- OR:
-
Odds ratio
- P:
-
Pertuzumab
- PROs:
-
Patient-reported outcomes
- qXw:
-
Every X weeks
- SD:
-
Standard deviation
- T:
-
Taxane
References
Richardson G, Dobish R (2007) Chemotherapy induced diarrhea. J Oncol Pharm Pract 13:181–198. https://doi.org/10.1177/1078155207077335
Stein A, Voigt W, Jordan K (2010) Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther Adv Medi Oncol 2:51–63. https://doi.org/10.1177/1758834009355164
Swain SM, Schneeweiss A, Gianni L, Gao JJ, Stein A, Waldron-Lynch M, Heeson S, Beattie MS, Yoo B, Cortes J, Baselga J (2017) Incidence and management of diarrhea in patients with HER2-positive breast cancer treated with pertuzumab. Ann Oncol 28:761–768. https://doi.org/10.1093/annonc/mdw695
Dang C, Iyengar N, Datko F, D’Andrea G, Theodoulou M, Dickler M, Goldfarb S, Lake D, Fasano J, Fornier M, Gilewski T, Modi S, Gajria D, Moynahan ME, Hamilton N, Patil S, Jochelson M, Norton L, Baselga J, Hudis C (2015) Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 33:442–447. https://doi.org/10.1200/jco.2014.57.1745
Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, CLEOPATRA Study Group (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Eng J Med 366:109–119. https://doi.org/10.1056/nejmoa1113216
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, McNally V, Ross G, Cortés J (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278–2284. https://doi.org/10.1093/annonc/mdt182
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J, Investigators ASC (2017) Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122–131. https://doi.org/10.1056/nejmoa1703643
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32. https://doi.org/10.1016/s1470-2045(11)70336-9
Tao G, Chityala PK (2021) Epidermal growth factor receptor inhibitor-induced diarrhea: Clinical incidence, toxicological mechanism, and management. Toxicol Res (Camb) 10:476–486. https://doi.org/10.1093/toxres/tfab026
Hirsh V, Blais N, Burkes R, Verma S, Croitoru K (2014) Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Current Oncol (Toronto, Ont) 21:329–336. https://doi.org/10.3747/co.21.2241
Pessi MA, Zilembo N, Haspinger ER, Molino L, Di Cosimo S, Garassino M, Ripamonti CI (2014) Targeted therapy-induced diarrhea: A review of the literature. Crit Rev Oncol Hematol 90:165–179. https://doi.org/10.1016/j.critrevonc.2013.11.008
Duan T, Cil O, Thiagarajah JR, Verkman AS (2019) Intestinal epithelial potassium channels and CFTR chloride channels activated in ErbB tyrosine kinase inhibitor diarrhea. JCI Insight 4:e126444. https://doi.org/10.1172/jci.insight.126444
Rugo HS, Di Palma JA, Tripathy D, Bryce R, Moran S, Olek E, Bosserman L (2019) The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat 175:5–15. https://doi.org/10.1007/s10549-018-05102-x
Kim Y, Quach A, Das S, Barrett KE (2020) Potentiation of calcium-activated chloride secretion and barrier dysfunction may underlie EGF receptor tyrosine kinase inhibitor-induced diarrhea. Physiol Rep 8:e14490. https://doi.org/10.14814/phy2.14490
Harada Y, Sekine H, Kubota K, Sadatomi D, Iizuka S, Fujitsuka N (2021) Calcium-activated chloride channel is involved in the onset of diarrhea triggered by EGFR tyrosine kinase inhibitor treatment in rats. Biomed Pharmacother 141:111860. https://doi.org/10.1016/j.biopha.2021.111860
Fischer H, Machen TE, Widdicombe JH, Carlson TJ, King SR, Chow JW, Illek B (2004) A novel extract SB-300 from the stem bark latex of Croton lechleri inhibits CFTR-mediated chloride secretion in human colonic epithelial cells. J Ethnopharmacol 93:351–357. https://doi.org/10.1016/j.jep.2004.04.005
Tradtrantip L, Namkung W, Verkman AS (2010) Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol 77:69–78. https://doi.org/10.1124/mol.109.061051
Macarthur RD, Hawkins TN, Brown SJ, Lamarca A, Clay PG, Barrett AC, Bortey E, Paterson C, Golden PL, Forbes WP (2013) Efficacy and safety of crofelemer for noninfectious diarrhea in HIV-seropositive individuals (ADVENT trial): a randomized, double-blind, placebo-controlled, two-stage study. HIV Clin Trials 14:261–273. https://doi.org/10.1310/hct1406-261
Frampton JE (2013) Crofelemer: a review of its use in the management of non-infectious diarrhoea in adult patients with HIV/AIDS on antiretroviral therapy. Drugs 73:1121–1129. https://doi.org/10.1007/s40265-013-0083-6
Cottreau J, Tucker A, Crutchley R, Garey KW (2012) Crofelemer for the treatment of secretory diarrhea. Expert Rev Gastroenterol Hepatol 6:17–23. https://doi.org/10.1586/egh.11.87
Patel TS, Crutchley RD, Tucker AM, Cottreau J, Garey KW (2013) Crofelemer for the treatment of chronic diarrhea in patients living with HIV/AIDS. HIV AIDS (Auckl) 15:153–162. https://doi.org/10.2147/hiv.s30948
Napo Pharmaceuticals Inc. (2020) MYTESI® (crofelemer). Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202292s007lbl.pdf. Accessed August 2022
Blake MR, Raker JM, Whelan K (2016) Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 44:693–703. https://doi.org/10.1111/apt.13746
US Department of Health and Human Services, National Institutes of Health, National Cancer Institute (2010) Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010). https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed Feb 2020
Benson AB 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, McCallum R, Mitchell EP, O’Dorisio TM, Vokes EE, Wadler S (2004) Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol 22:2918–2926. https://doi.org/10.1200/jco.2004.04.132
O’Donnell LJ, Virjee J, Heaton KW (1990) Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ (Clinical research ed) 300:439–440. https://doi.org/10.1136/bmj.300.6722.439
Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32:920–924. https://doi.org/10.3109/00365529709011203
Gao JJ, Tan M, Pohlmann PR, Swain SM (2017) HALT-D: A phase II evaluation of crofelemer for the prevention and prophylaxis of diarrhea in patients with breast cancer on pertuzumab-based regimens. Clin Breast Cancer 17:76–78. https://doi.org/10.1016/j.clbc.2016.08.005
Skarke C, Jarrar M, Schmidt H, Kauert G, Langer M, Geisslinger G, Lötsch J (2003) Effects of ABCB1 (multidrug resistance transporter) gene mutations on disposition and central nervous effects of loperamide in healthy volunteers. Pharmacogenetics 13:651–660. https://doi.org/10.1097/00008571-200311000-00001
Okura T, Morita Y, Ito Y, Kagawa Y, Yamada S (2010) Effects of quinidine on antinociception and pharmacokinetics of morphine in rats. J Pharm Pharmacol 61:593–597. https://doi.org/10.1211/jpp.61.05.0007
Barcenas CH, Hurvitz SA, Di Palma JA, Bose R, Chien AJ, Iannotti N, Marx G, Brufsky A, Litvak A, Ibrahim E, Alvarez RH, Ruiz-Borrego M, Chan N, Manalo Y, Kellum A, Trudeau M, Thirlwell M, Garcia Saenz J, Hunt D, Bryce R, McCulloch L, Rugo HS, Tripathy D, Chan A, CONTROL Study Investigators (2020) Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: The CONTROL trial. Ann Oncol 31:1223–1230. https://doi.org/10.1016/j.annonc.2020.05.012
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW, Wood WC, Davidson NE (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671. https://doi.org/10.1056/nejmoa0707056
Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L, Lower E, Laufman L, Sundaram S, Urba WJ, Pritchard KI, Mennel R, Richards D, Olsen S, Meyers ML, Ravdin PM (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. Journal Clin Oncol 23:5542–5551. https://doi.org/10.1200/jco.2005.02.027
Bossi P, Antonuzzo A, Cherny NI, Rosengarten O, Pernot S, Trippa F, Schuler U, Snegovoy A, Jordan K, Ripamonti CI, Guidelines Committee ESMO (2018) Diarrhoea in adult cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 29:IV126–IV142. https://doi.org/10.1093/annonc/mdy145
Michael M, Brittain M, Nagai J, Feld R, Hedley D, Oza A, Siu L, Moore MJ (2004) Phase II study of activated charcoal to prevent irinotecan-induced diarrhea. J. Clin Oncol 22:4410–4417. https://doi.org/10.1200/jco.2004.11.125
Karthaus M, Ballo H, Abenhardt W, Steinmetz T, Geer T, Schimke J, Braumann D, Behrens R, Behringer D, Kindler M, Messmann H, Boeck HP, Greinwald R, Kleeberg U (2005) Prospective, double-blind, placebo-controlled, multicenter, randomized phase III study with orally administered budesonide for prevention of irinotecan (CPT-11)-induced diarrhea in patients with advanced colorectal cancer. Oncology 68:326–332. https://doi.org/10.1159/000086971
Acknowledgments
This study was supported by Genentech, Inc., a member of the Roche group (funding), and Napo Pharmaceuticals, Inc. (provided crofelemer). This research was supported by the Biostatistics and Biomedical Informatics Shared Resource of the Georgetown Lombardi Comprehensive Cancer Center (P30-CA051008). Napo Pharmaceuticals, Inc. acknowledges the contributions of the indigenous people of the Northwest Amazonian region in the development of crofelemer. We would also like to acknowledge Dr Jennifer Gao who participated in the design of the study during her National Cancer Institute oncology fellowship. Support for third-party editorial assistance for this manuscript, furnished by Daniel Clyde, PhD, of Health Interactions, was provided by Genentech, Inc.
Funding
This work was supported by Genentech, Inc., a member of the Roche group (Funding), Napo Pharmaceuticals, Inc. (provided crofelemer), and the Biostatistics and Biomedical Informatics Shared Resource of the Georgetown Lombardi Comprehensive Cancer Center (Grant Number NIH P30-CA051008). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study conception and design was done by SMS. Material preparation was done by PRP, NS, and SMS. All authors contributed to the data acquisition. Data analysis was performed by TW, MT, NS, PRP, and SMS. The first draft of the manuscript was written by PRP, and all authors commented on at least one draft of the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
Paula R. Pohlmann reports honoraria from the FRONTIERS Editorial Board; consulting/advisory board roles for Personalized Cancer Therapy (Perthera), Immunonet BioSciences, Sirtex, Xcenda/Sirtex, CARIS, Pfizer, Heron, Puma, BOLT Therapeutics, and AbbVie; lectures for Genentech/Roche, Dava Oncology, ASCO Courses, ION Oral Oncolytics, and OncLive; research funding to her institution from Pfizer, Genentech/Roche, Pieris, Fabre-Kramer, BOLT, Byondis, and SEAGEN; and patents (United States Patent no. 9,745,377; 8,501,417; 8,486,413; 9,023,362; patent application CTC-CRC). Deena Graham reports research funding to her institute from Genentech, MedStar Health, and Napo. Tianmin Wu reports research funding to her institution from Genentech through MedStar Health. Yvonne Ottaviano reports research funding to her institution from Genentech through MedStar Health and study drug supply from Napo. Mahsa Mohebtash reports research funding to her institution from Genentech through MedStar Health and study drug supply from Napo. Shweta Kurian reports research funding to her institution from Genentech through MedStar Health and study drug supply from Napo. Donna McNamara reports research funding to her institution from Genentech through MedStar Health and study drug supply from Napo. Filipa Lynce has consulted for AstraZeneca and Daiichi Sankyo and has received research funding to her institution from AstraZeneca, CytomX, and Zentalis. Robert Warren reports research funding to his institution from Genentech. Asma Dilawari has served on the Paxman Scalp Cooling Research Advisory Committee but did not receive monetary compensation and reports research funding to her institution to conduct the study from Genentech. Suman Rao reports funding to her institution from Genentech. Candace Mainor reports research funding to her institution from Genentech through MedStar Health and study drug supply from Napo. Nicole Swanson reports research funding to her institution from Genentech (funding source) and study drug supply by Napo Pharmaceuticals. Ming Tan reports research funding to his institution from Genentech through MedStar Health. Claudine Isaacs reports consultancies for Genentech, Seattle Genetics, and PUMA. Sandra M. Swain reports grants/contracts to her institution from Breast Cancer Research Foundation, Genentech/Roche, and Kailos Genetics; consulting fees from AstraZeneca, Daiichi Sankyo, Molecular Templates, Silverback Therapeutics, Eli Lilly, Merck, Natera, Exact Sciences, Athenex, Biotheranostics, and Genentech/Roche; non-promotional speaking fees from Genentech/Roche, Daiichi Sankyo, and Beijing Medical Foundation; travel support from Genentech/Roche, Caris, and Daiichi Sankyo; serving on a data safety and monitoring board for AstraZeneca; and leadership or a fiduciary role for ASCO Conquer Cancer Foundation and The National Surgical Adjuvant Breast and Bowel Project Foundation (all outside of the submitted work). All authors received third-party medical writing support from Genentech, Inc.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Georgetown University Institutional Review Board on 12/09/2015 under IRB # 2015–0547.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent to publish
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pohlmann, P.R., Graham, D., Wu, T. et al. HALT-D: a randomized open-label phase II study of crofelemer for the prevention of chemotherapy-induced diarrhea in patients with HER2-positive breast cancer receiving trastuzumab, pertuzumab, and a taxane. Breast Cancer Res Treat 196, 571–581 (2022). https://doi.org/10.1007/s10549-022-06743-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06743-9
Keywords
Profiles
- Paula R. Pohlmann View author profile