Abstract
Purpose
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer, characterized by substantial risks of early disease recurrence and mortality. We constructed and validated clinical calculators for predicting recurrence-free survival (RFS) and overall survival (OS) for TNBC.
Methods
Data from 605 women with centrally confirmed TNBC who underwent primary breast cancer surgery at Mayo Clinic during 1985–2012 were used to train risk models. Variables included age, menopausal status, tumor size, nodal status, Nottingham grade, surgery type, adjuvant radiation therapy, adjuvant chemotherapy, Ki67, stromal tumor-infiltrating lymphocytes (sTIL) score, and neutrophil-to-lymphocyte ratio (NLR). Final models were internally validated for calibration and discrimination using ten-fold cross-validation and compared with their base-model counterparts which include only tumor size and nodal status. Independent external validation was performed using data from 478 patients diagnosed with stage II/III invasive TNBC during 1986–1992 in the British Columbia Breast Cancer Outcomes Unit database.
Results
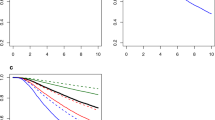
Final RFS and OS models were well calibrated and associated with C-indices of 0.72 and 0.73, as compared with 0.64 and 0.62 of the base models (p < 0.001). In external validation, the discriminant ability of the final models was comparable to the base models (C-index: 0.59–0.61). The RFS model demonstrated greater accuracy than the base model both overall and within patient subgroups, but the advantages of the OS model were less profound.
Conclusions
This TNBC clinical calculator can be used to predict patient outcomes and may aid physician’s communication with TNBC patients regarding their long-term disease outlook and planning treatment strategies.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Centers for Disease Control and Prevention (2019) Breast cancer statistics. https://www.cdc.gov/cancer/breast/statistics/
Copson ER, Maishman TC, Tapper WJ et al (2018) Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 19:169–180
Couch FJ, Shimelis H, Hu C et al (2017) Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol 3:1190–1196
Dietze EC, Sistrunk C, Miranda-Carboni G et al (2015) Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer 15:248–254
Perou CM, Sørlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767
Purrington KS, Visscher DW, Wang C et al (2016) Genes associated with histopathologic features of triple negative breast tumors predict molecular subtypes. Breast Cancer Res Treat 157:117–131
Prat A, Adamo B, Cheang MC et al (2012) Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 18:123–133
AJCC Cancer Staging Manual (2017) 8th edn. Springer, New York
University of Cambridge. Predict Tool Version 2.0: Breast Cancer Overall Survival, https://breast.predict.nhs.uk/predict_v2.0.html
Wishart GC, Azzato EM, Greenberg DC et al (2010) PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 12(1):R1
Wishart GC, Bajdik CD, Azzato EM et al (2011) A population-based validation of the prognostic model PREDICT for early breast cancer. Eur J Surg Oncol 37:411–417
Harnan S, Tappenden P, Cooper K et al (2019) Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer: a systematic review and economic analysis. Health Technol Assess 23(30):1–328
Ovcaricek T, Frkovic SG, Matos E et al (2011) Triple negative breast cancer—prognostic factors and survival. Radiol Oncol 45:46–52
Urru SAM, Gallus S, Bosetti C et al (2018) Clinical and pathological factors influencing survival in a large cohort of triple-negative breast cancer patients. BMC Cancer 18(1):56
Steward L, Conant L, Gao F et al (2014) Predictive factors and patterns of recurrence in patients with triple negative breast cancer. Ann Surg Oncol 21:2165–2171
Loi S, Sirtaine N, Piette F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 31:860–867
Loi S, Michiels S, Salgado R et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25(8):1544–1550
Adams S, Gray RJ, Demaria S et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966
Pruneri G, Vingiani A, Bagnardi V et al (2016) Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol 27(2):249–256
Geng SK, Fu SM, Fu YP et al (2018) Neutrophil to lymphocyte ratio is a prognostic factor for disease free survival in patients with breast cancer underwent curative resection. Medicine (Baltimore) 97(35):e11898
Chae S, Kang KM, Kim HJ et al (2018) Neutrophil-lymphocyte ratio predicts response to chemotherapy in triple-negative breast cancer. Curr Oncol 25(2):e113–e119
Patel DA, Xi J, Luo J et al (2019) Neutrophil-to-lymphocyte ratio as a predictor of survival in patients with triple-negative breast cancer. Breast Cancer Res Treat 174(2):443–452
Pistelli M, De Lisa M, Ballatore Z et al (2015) Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer 15:195
Leon-Ferre RA, Polley MY, Liu H et al (2018) Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat 167(1):89–99
Loi S, Drubay D, Adams S et al (2019) Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 37(7):559–569
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271
Hudis CA, Barlow WE, Costantino JP et al (2007) Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25(15):2127–2132
Cheng AS, Leung SCY, Gao D et al (2020) Mismatch repair protein loss in breast cancer: clinicopathological associations in a large British Columbia cohort. Breast Cancer Res Treat 179(1):3–10
Burugu S, Gao D, Leung S et al (2018) TIM-3 expression in breast cancer. Oncoimmunology 7(11):e1502128
Cheang MC, Voduc D, Bajdik C et al (2008) Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 14(5):1368–1376
Cheang MC, Chia SK, Voduc D et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750
Cheang MC, Treaba DO, Speers CH et al (2006) Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol 24(36):5637–5644
Little R, Rubin D (2002) Statistical analysis with missing data, 2nd edn. Wiley-Interscience, New York
Harrell F (2001) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer, New York
Steyerberg E (2009) Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer, New York
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Heagerty PJ, Lumley T, Pepe MS (2000) Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56:337–344
R Core Team (2008) R: A language and environment for statistical computing, http://www.R-project.org/
Murphy BL, Day CN, Hoskin TL et al (2018) Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtype. Ann Surg Oncol 25:2241–2248
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Masuda N, Lee SJ, Ohtani S et al (2017) Adjuvant Capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376(22):2147–2159
von Minckwitz G, Huang CS, Mano MS et al (2019) Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 380(7):617–628
Funding
Research reported in this article was supported by the National Cancer Institute of the National Institutes of Health under Award Number P50 CA116201 (Mayo Clinic Breast Cancer Specialized Program of Research Excellence) and the Breast Cancer Research Foundation (BCRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Torsten O. Nielsen holds a proprietary interest in the Prosigna breast cancer assay through Bioclassifier LLC, licensed to Veracyte, Inc. Dr. Goetz reports personal fees from Genomic Health, consulting fees from Lilly, Biovica, Novartis, Sermonix, Context Pharm, Pfizer, Biotheranostics, and grants from Pfizer, Lilly, and Sermonix. Other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The need for obtaining informed consent was waived by the institutional review board, given that this study was retrospective and non-interventional.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Polley, MY.C., Leon-Ferre, R.A., Leung, S. et al. A clinical calculator to predict disease outcomes in women with triple-negative breast cancer. Breast Cancer Res Treat 185, 557–566 (2021). https://doi.org/10.1007/s10549-020-06030-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-06030-5
Keywords
Profiles
- Jason Sinnwell View author profile