Abstract
Purpose
Obtaining tumor-free surgical margins is critical to prevent recurrence in breast-conserving surgery but it remains challenging. We assessed the LUM Imaging System for real-time, intraoperative detection of residual tumor.
Methods
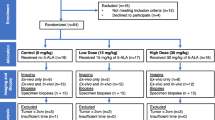
Lumpectomy cavity walls and excised specimens of breast cancer lumpectomy patients were assessed with the LUM Imaging System (Lumicell, Inc., Wellesley MA) with and without intravenous LUM015, a cathepsin-activatable fluorescent agent. Fluorescence at potential sites of residual tumor was evaluated with a sterile hand-held probe, displayed on a monitor and correlated with histopathology.
Results
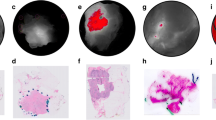
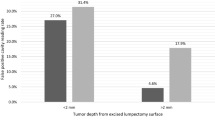
Background autofluorescence was assessed in excised specimens from 9 patients who did not receive LUM015. In vivo lumpectomy cavities and excised specimens were then imaged in 15 women undergoing breast cancer surgery who received no LUM015, 0.5, or 1 mg/kg LUM015 (5 women per dose). Among these, 11 patients had invasive carcinoma with ductal carcinoma in situ (DCIS) and 4 had only DCIS. Image acquisition took 1 s for each 2.6-cm-diameter surface. No significant background normal breast fluorescence was identified. Elevated fluorescent signal was seen from invasive cancers and DCIS. Mean tumor-to-normal signal ratios were 4.70 ± 1.23 at 0.5 mg/kg and 4.22 ± 0.9 at 1.0 mg/kg (p = 0.54). Tumor was distinguished from normal tissue in pre-and postmenopausal women and readings were not affected by breast density. Some benign tissues produced fluorescent signal with LUM015.
Conclusion
The LUM Imaging System allows rapid identification of residual tumor in the lumpectomy cavity of breast cancer patients and may reduce rates of positive margins.



Similar content being viewed by others
References
Fisher B, Anderson S, John Bryant J et al (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241. https://doi.org/10.1056/NEJMoa022152
Veronesi U, Cascinelli N, Mariani L et al (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347(16):1227–1232
Arvold ND, Taghian AG, Niemierko A et al (2011) Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol 29:3885–3891
Clarke M, Collins R, Darby S et al. (2005) Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366:2087–2106
Merrill AL, Buckley J, Tang R, Brachtel E, Rai U, Michaelson J, Ly A, Specht MC, Yagi Y, Smith BL (2017) A study of the growth patterns of breast carcinoma using 3D reconstruction: a pilot study. Breast J 23:83–89. https://doi.org/10.1111/tbj.12688
Esbona K, Li Z, Wilke L (2012) Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann Surg Oncol 19:3236–3245. https://doi.org/10.1245/s10434-012-2492-2
Coopey SB, Smith BL, Hanson SA, Buckley JM, Hughes KS, Gadd MA, Specht MC (2011) The safety of multiple re-excisions after lumpectomy for breast cancer. Ann Surg Oncol 18:3797–3701
Chagpar AB, Brigid K. Killelea BK, Tsangaris TN et al (2015) A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med 373:503–510. https://doi.org/10.1056/NEJMoa1504473
Coopey SB, Buckley JM, Smith BL et al (2011) Lumpectomy cavity shaved margins do not impact re excision rates in breast cancer patients. Ann Surg Oncol 18:3036–3040. https://doi.org/10.1245/s10434-011-1909-7
McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, Engel JM, Onitilo AA (2012) Variability in reexcision following breast conservation surgery. JAMA 307(5):467–475. https://doi.org/10.1001/jama.2012.43
O’Kelly Priddy CM, Forte VA, Lang JE (2016) The importance of surgical margins in breast cancer. J Surg Oncol 113:256–263
Riedle O, Fitzal F, Mader N et al (2009) Intraoperative frozen section analysis for breast-conserving therapy in 1016 patients with breast cancer. Eur J Surg Oncol 35:264–270
Cabioglu N, Hunt KK, Sahin AA et al (2007) Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol 14:1458–1471
Tang R, Buckley JM, Fernandez L, Aftreth O, Michaelson J, Saksena M, Coopey S, Specht M, Gadd M, Yagi Y, Rafferty E, Brachtel E, Smith BL (2013) Micro-computed tomography (Micro-CT): a novel method for intra-operative breast cancer specimen imaging. Breast Cancer Res Treat 139:311–316
Tang R, Saksena M, Coopey SB, Fernandez L, Buckley JM, Lei L, Aftreth O, Koerner F, Michaelson J, Rafferty E, Brachtel E, Smith BL (2016) Intraoperative micro-computed tomography (micro-CT): a novel method for determination of primary tumour dimensions in breast cancer specimens. Br J Radiol 89(1058):20150581. https://doi.org/10.1259/bjr.20150581
Brachtel EF, Johnson NB, Huck AE, Rice-Stitt TL, Vangel MG, Smith BL, Tearney GJ, Kang D (2016) Spectrally encoded confocal microscopy for diagnosing breast cancer in excision and margin specimens. Lab Invest 96:459–467. https://doi.org/10.1038/labinvest.2015.158
Ngyuyen FT, Zysk AM, Chaney EJ et al (2009) Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res 69:8790–8796. https://doi.org/10.1158/0008-5472.CAN-08-4340
Schnabel F, Boolbol SK, Gittleman M et al (2014) A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol 21:1589–1595. https://doi.org/10.1245/s10434-014-3602-0
Allweis TM, Kaufman Z, Lelcuk S et al (2008) A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg 196:483–489. https://doi.org/10.1016/j.amjsurg.2008.06.024
Tang R, Coopey SB, Specht MC, Lei L, Gadd MA, Hughes KS, Brachtel EF, Smith BL (2015) Lumpectomy specimen margins are not reliable in predicting residual disease in patients undergoing breast conserving surgery. Am J Surg 210:93–98
Whitley MJ, Cardona DM, Lazarides AL et al. (2016) A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aad0293
Mito JK, Ferrer JM, Brigman BE, et al (2012) Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging. Cancer 118:5320–5330
World Health Organization Classification of Tumours of the Breast, Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (eds) (2012) International Agency for Research on Cancer, Lyon
Harness JK, Guiliano AE, Pockaj BA et al. (2014) Margins: a status report from the annual meeting of the American Society of Breast Surgeons. Ann Surg Oncol 21:3192–3197
Demos SG, Gandour-Edwards R, Ramsamooj R, White RD (2004) Near-infrared autofluorescence imaging for detection of cancer. J Biomed Opt 9:587–592
Sharma V, Shivalingaiah S, Peng Y et al (2012) Auto-fluorescence lifetime and light reflectance spectroscopy for breast cancer diagnosis: potential tools for intraoperative margin detection. Biomed Opt Express 3:1825–1840. https://doi.org/10.1364/BOE.3.001825
Funding
This study was supported by NCI Grant 1R21CA173762-01. We gratefully acknowledge the Massachusetts General Hospital Translational Clinical Research Center and their NIH Grant #1UL1TR001102 for providing research nurse support. The clinical production of LUM015 was funded with federal funds from the National Cancer Institute, National Institutes of Health, under NCI’s Experimental Therapeutics Program (http://www.next.cancer.gov).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors D. Strasfeld and J. Ferrer have received remuneration and stock ownership from Lumicell, Inc. All other authors declare that he/she have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Smith, B.L., Gadd, M.A., Lanahan, C.R. et al. Real-time, intraoperative detection of residual breast cancer in lumpectomy cavity walls using a novel cathepsin-activated fluorescent imaging system. Breast Cancer Res Treat 171, 413–420 (2018). https://doi.org/10.1007/s10549-018-4845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4845-4