Abstract
Aim
To investigate the clinical and prognostic significance of circulated tumor cells (CTC) marked by cytokeratin 19 coding gene KRT19 mRNA and carcinoembryonic antigen coding gene CEACAM5 mRNA in preoperative peripheral blood of breast cancer patients and provide molecular markers for breast cancer metastasis risk.
Methods
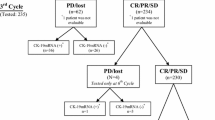
The mRNA levels of KRT19 and CEACAM5 in preoperative peripheral blood of breast cancer patients without (n = 603) and with (n = 76) distant metastases at the time of initial diagnosis were detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The relationship between CTCKRT19, CTCCEACAM5 and clinicopathological features, local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), or overall survival (OS) was statistically analyzed.
Results
In different pathological stages of breast cancer, the rates of CTCKRT19−pos and CTCCEACAM5−pos increased with the increase of the stages (P = 0.077 and P = 0.004). Preoperative CTCKRT19−pos in breast cancer patients was closely related to the lymph node metastasis statues (P < 0.0001), and had no significant correlation with other clinicopathological features. There was no significant correlation between CTCCEACAM5 and the clinicopathological features. Patients with high levels of CTC double-marked by KRT19 and CEACAM5 mRNA had shorter DMFS (P < 0.0001) and OS (P = 0.016) for patients with breast cancer. The 7-year DMFS rates for the low-, intermediate-, and high-risk groups were 90.7%, 67.5%, and 59.1%, respectively (P < 0.0001). The prognosis of patients with decreased KRT19 and CEACAM5 mRNA after treatment is better than that of patients who have not decreased, and the combination of the two indicators is better than the single one for predicting PFS (P = 0.002 compare with P = 0.036 or P = 0.047).
Conclusion
Double-marked CTC by KRT19 and CEACAM5 mRNA is a prognostic index of breast cancer patients before surgery and after chemotherapy. Single-marked CTC by KRT19 mRNA indicates lymph node statues of preoperative patients. Therefore, the RT-qPCR-based molecular diagnosis of CTC could be used for prognostic prediction of breast cancer patients and guiding clinical treatment.






Similar content being viewed by others
References
Howell A, Anderson AS, Clarke RB, Duffy SW, Evans DG, Garcia-Closas M, Gescher AJ, Key TJ, Saxton JM, Harvie MN (2014) Risk determination and prevention of breast cancer. Breast Cancer Res 16:446. https://doi.org/10.1186/s13058-014-0446-2
Yates LR, Knappskog S, Wedge D, JHR F, Gonzalez S, Martincorena I, Alexandrov LB, Van Loo P, Haugland HK, Lilleng PK, Gundem G, Gerstung M, Pappaemmanuil E, Gazinska P, Bhosle SG, Jones D, Raine K, Mudie L, Latimer C, Sawyer E, Desmedt C, Sotiriou C, Stratton MR, Sieuwerts AM, Lynch AG, Martens JW, Richardson AL, Tutt A, Lønning PE, Campbell PJ (2017) Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32:169–184.e7. https://doi.org/10.1016/j.ccell.2017.07.005
Bednarz-Knoll N, Alix-Panabières C, Pantel K (2011) Clinical relevance and biology of circulating tumor cells. Breast Cancer Res 13:228. https://doi.org/10.1186/bcr2940
Haber DA, Velculescu VE (2014) Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 4:650–661. https://doi.org/10.1158/2159-8290.CD-13-1014
Lowes LE, Bratman SV, Dittamore R, Done S, Kelley SO, Mai S, Morin RD, Wyatt AW, Allan AL (2016) Circulating tumor cells (CTC) and cell-free DNA (cfDNA) Workshop 2016: scientific opportunities and logistics for cancer clinical trial incorporation. Int J Mol Sci. https://doi.org/10.3390/ijms17091505
Alix-Panabières C, Pantel K (2014) Challenges in circulating tumour cell research. Nat Rev Cancer 14:623–631. https://doi.org/10.1038/nrc3820
Peeters DJ, van Dam PJ, Van den Eynden GG, Rutten A, Wuyts H, Pouillon L, Peeters M, Pauwels P, Van Laere SJ, van Dam PA, Vermeulen PB, Dirix LY (2014) Detection and prognostic significance of circulating tumour cells in patients with metastatic breast cancer according to immunohistochemical subtypes. Br J Cancer 110:375–383. https://doi.org/10.1038/bjc.2013.743
Wallwiener M, Riethdorf S, Hartkopf AD, Modugno C, Nees J, Madhavan D, Sprick MR, Schott S, Domschke C, Baccelli I, Schönfisch B, Burwinkel B, Marmé F, Heil J, Sohn C, Pantel K, Trumpp A, Schneeweiss A (2014) Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer 14:512. https://doi.org/10.1186/1471-2407-14-512
Saloustros E, Perraki M, Apostolaki S, Kallergi G, Xyrafas A, Kalbakis K, Agelaki S, Kalykaki A, Georgoulias V, Mavroudis D (2011) Cytokeratin-19 mRNA-positive circulating tumor cells during follow-up of patients with operable breast cancer: prognostic relevance for late relapse. Breast Cancer Res 13:R60. https://doi.org/10.1186/bcr2897
Terrenato I, D’Alicandro V, Casini B, Perracchio L, Rollo F, De Salvo L, Di FS, Di FF, Pescarmona E, Maugeri-Saccà M, Mottolese M, Buglioni S (2017) A cut-off of 2150 cytokeratin 19 mRNA copy number in sentinel lymph node may be a powerful predictor of non-sentinel lymph node status in breast cancer patients. PLoS ONE 12:e0171517. https://doi.org/10.1371/journal.pone.0171517
Zhao S, Mei Y, Wang Y, Zhu J, Zheng G, Ma R (2015) Levels of CEA, CA153, CA199, CA724 and AFP in nipple discharge of breast cancer patients. Int J Clin Exp Med 8:20837–20844
Hioki T, Sugimura Y (1999) Detection of circulating cancer cells by nested reverse transcription-polymerase chain reaction of cytokeratin-19 in patients with renal cell carcinoma. Hinyokika Kiyo 45:577–581
Andergassen U, Hofmann S, Kölbl AC, Schindlbeck C, Neugebauer J, Hutter S, Engelstädter V, Ilmer M, Friese K, Jeschke U (2013) Detection of tumor cell-specific mRNA in the peripheral blood of patients with breast cancer—evaluation of several markers with real-time reverse transcription-PCR. Int J Mol Sci 14:1093–1104
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474. https://doi.org/10.1245/s10434-010-0985-4
Scott A, Ambannavar R, Jeong J, Liu ML, Cronin MT (2011) RT-PCR-based gene expression profiling for cancer biomarker discovery from fixed, paraffin-embedded tissues. Methods Mol Biol 724:239–257. https://doi.org/10.1007/978-1-61779-055-3_15
Shao Y, Sun X, He Y, Liu C, Liu H (2015) Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS ONE 10:e0133830. https://doi.org/10.1371/journal.pone.0133830
Yang Y, Zhang H, Zhang M, Meng Q, Cai L, Zhang Q (2017) Elevation of serum CEA and CA15-3 levels during antitumor therapy predicts poor therapeutic response in advanced breast cancer patients. Oncol Lett 14:7549–7556. https://doi.org/10.3892/ol.2017.7164
Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Ch K, Apostolaki S, Malamos N, Kakolyris S, Kotsakis A, Xenidis N, Reppa D, Georgoulias V (2002) Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol 20:3404–3412. https://doi.org/10.1200/JCO.2002.08.135
Park HS, Han HJ, Lee S, Kim GM, Park S, Choi YA, Lee JD, Kim GM, Sohn J, Kim SI (2017) Detection of circulating tumor cells in breast cancer patients using cytokeratin-19 real-time RT-PCR. Yonsei Med J 58:19–26. https://doi.org/10.3349/ymj.2017.58.1.19
Imamura M, Morimoto T, Nomura T, Michishita S, Nishimukai A, Higuchi T, Fujimoto Y, Miyagawa Y, Kira A, Murase K, Araki K, Takatsuka Y, Oh K, Masai Y, Akazawa K, Miyoshi Y (2018) Independent prognostic impact of preoperative serum carcinoembryonic antigen and cancer antigen 15-3 levels for early breast cancer subtypes. World J Surg Oncol 16:26. https://doi.org/10.1186/s12957-018-1325-6
Yu XF, Yang HJ, Lei L, Wang C, Huang J (2016) CK19 mRNA in blood can predict non-sentinel lymph node metastasis in breast cancer. Oncotarget 7:30504–30510. https://doi.org/10.18632/oncotarget.8851
Qiao YF, Chen CG, Yue J, Ma MQ, Ma Z, Yu ZT (2017) Prognostic significance of preoperative and postoperative CK19 and CEA mRNA levels in peripheral blood of patients with gastric cardia cancer. World J Gastroenterol 23:1424–1433. https://doi.org/10.3748/wjg.v23.i8.1424
Qiao YF, Chen CG, Yue J, Ma Z, Yu ZT (2016) Clinical significance of preoperative and postoperative cytokeratin 19 messenger RNA level in peripheral blood of esophageal cancer patients. Dis Esophagus 29:929–936. https://doi.org/10.1111/dote.12403
Yang XR, Xu Y, Shi GM, Fan J, Zhou J, Ji Y, Sun HC, Qiu SJ, Yu B, Gao Q, He YZ, Qin WZ, Chen RX, Yang GH, Wu B, Lu Q, Wu ZQ, Tang ZY (2008) Cytokeratin 10 and cytokeratin 19: predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res 14:3850–3859. https://doi.org/10.1158/1078-0432.CCR-07-4338
Ge MJ, Wu QC, Wang M, Zhang YH, Li LB (2005) Detection of disseminated lung cancer cells in regional lymph nodes by assay of CK19 reverse transcriptase polymerase chain reaction and its clinical significance. J Cancer Res Clin Oncol 131:662–668. https://doi.org/10.1007/s00432-005-0009-0
Zhang Q, Qu H, Sun G, Li Z, Ma S, Shi Z, Zhao E, Zhang H, He Q (2017) Early postoperative tumor marker responses provide a robust prognostic indicator for N3 stage gastric cancer. Medicine (Baltimore) 96:e7560. https://doi.org/10.1097/MD.0000000000007560
Acknowledgements
The authors would like to thank Dr. Yumei Feng (Tianjin Medical University Cancer Research Institute and Hospital Department of Biochemistry and Molecular Biology, Tianjin, China) for the useful discussion and insightful observation during the research process. Thanks to Tianjin Medical University Cancer Research Institute and Hospital laboratory of Biochemistry and Molecular Biology for technical assistance in RT-qPCR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the respective institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wang, XM., Zhang, Z., Pan, LH. et al. KRT19 and CEACAM5 mRNA-marked circulated tumor cells indicate unfavorable prognosis of breast cancer patients. Breast Cancer Res Treat 174, 375–385 (2019). https://doi.org/10.1007/s10549-018-05069-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-05069-9