Abstract
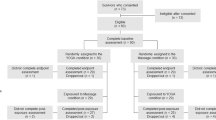
Up to 50 % of breast cancer survivors on aromatase inhibitor therapy report musculoskeletal symptoms such as joint and muscle pain, significantly impacting treatment adherence and discontinuation rates. We conducted a secondary data analysis of a nationwide, multi-site, phase II/III randomized, controlled, clinical trial examining the efficacy of yoga for improving musculoskeletal symptoms among breast cancer survivors currently receiving hormone therapy (aromatase inhibitors [AI] or tamoxifen [TAM]). Breast cancer survivors currently receiving AI (N = 95) or TAM (N = 72) with no participation in yoga during the previous 3 months were randomized into 2 arms: (1) standard care monitoring and (2) standard care plus the 4-week yoga intervention (2x/week; 75 min/session) and included in this analysis. The yoga intervention utilized the UR Yoga for Cancer Survivors (YOCAS©®) program consisting of breathing exercises, 18 gentle Hatha and restorative yoga postures, and meditation. Musculoskeletal symptoms were assessed pre- and post-intervention. At baseline, AI users reported higher levels of general pain, muscle aches, and total physical discomfort than TAM users (all P ≤ 0.05). Among all breast cancer survivors on hormonal therapy, participants in the yoga group demonstrated greater reductions in musculoskeletal symptoms such as general pain, muscle aches and total physical discomfort from pre- to post-intervention than the control group (all P ≤ 0.05). The severity of musculoskeletal symptoms was higher for AI users compared to TAM users. Among breast cancer survivors on hormone therapy, the brief community-based YOCAS©® intervention significantly reduced general pain, muscle aches, and physical discomfort.
Similar content being viewed by others
References
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, Forbes JF, Smith I, Lang I, Wardley A et al (2011) Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol 12:1101–1108. doi:10.1016/S1470-2045(11)70270-4
Arimidex TAoiCTG, Forbes JF, Cuzick J, Buzdar A, Howell A, JS Tobias, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100 month analysis of the ATAC trial. Lancet Oncol 9:45–53. doi:10.1016/S1470-2045(07)70385-6
International Breast Cancer Study G, Group BIGC, Regan MM, Price KN, Giobbie-Hurder A, Thurlimann B, Gelber RD (2011) Interpreting breast international group (big) 1-98: a randomized, double-blind, phase iii trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive, early breast cancer. Breast Cancer Res 13:209. doi:10.1186/bcr2837
Mouridsen HT (2006) Incidence and management of side effects associated with aromatase inhibitors in the adjuvant treatment of breast cancer in postmenopausal women. Curr Med Res Opin 22:1609–1621. doi:10.1185/030079906X115667
Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98:1802–1810
Buzdar A, Howell A, Cuzick J, Wale C, Distler W, Hoctin-Boes G, Houghton J, Locker GY, Nabholtz JM (2006) Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 7:633–643. doi:10.1016/S1470-2045(06)70767-7
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M et al (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 25:486–492. doi:10.1200/JCO.2006.08.8617
Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, Smith I, Mauriac L, Forbes JF, Price KN, Regan MM et al (2009) Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 361:766–776. doi:10.1056/NEJMoa0810818
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883. doi:10.1200/JCO.2007.10.7573
Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, Greene BT, DeMichele A (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115:3631–3639. doi:10.1002/cncr.24419
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P et al (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111:365–372. doi:10.1007/s10549-007-9774-6
Oberguggenberger A, Hubalek M, Sztankay M, Meraner V, Beer B, Oberacher H, Giesinger J, Kemmler G, Egle D, Gamper EM et al (2011) Is the toxicity of adjuvant aromatase inhibitor therapy underestimated? Complementary information from patient-reported outcomes (PROs). Breast Cancer Res Treat 128:553–561. doi:10.1007/s10549-011-1378-5
Group AT, Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, Buzdar AU (2008) Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol 9:866–872. doi:10.1016/S1470-2045(08)70182-7
Blencowe NS, Reichl C, Gahir J, Paterson I (2010) The use of Nolvadex in the treatment of generic tamoxifen-associated small joint arthralgia. Breast 19:243–245. doi:10.1016/j.breast.2010.02.004
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562. doi:10.1200/JCO.2007.11.5451
Dent S, DiValentin T, Vandermeer L, Spaans J, Verma S (2006) Long term toxicities in women with early stage breast cancer treated with aromatase inhibitors: data from a tertiary care center. Breast Cancer Res Treat 100(suppl 1):S190
Fontaine C, Meulemans A, Huizing M, Collen C, Kaufman L, De Mey J, Bourgain C, Verfaillie G, Lamote J, Sacre R et al (2008) Tolerance of adjuvant letrozole outside of clinical trials. Breast 17:376–381. doi:10.1016/j.breast.2008.02.006
Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, Ebrahimi B, Yeon C, Howard F (2007) Aromatase inhibitor-associated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer 7:775–778. doi:10.3816/CBC.2007.n.038
Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529–537. doi:10.1007/s10549-010-1132-4
Ziller V, Kalder M, Albert US, Holzhauer W, Ziller M, Wagner U, Hadji P (2009) Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann oncol 20:431–436. doi:10.1093/annonc/mdn646
McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, Fahey TP (2008) Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 99:1763–1768. doi:10.1038/sj.bjc.6604758
Ulger O, Yagli NV (2010) Effects of yoga on the quality of life in cancer patients. Complement Ther Clin Pract 16:60–63. doi:10.1016/j.ctcp.2009.10.007
Galantino ML, Greene L, Archetto B, Baumgartner M, Hassall P, Murphy JK, Umstetter J, Desai K (2012) A qualitative exploration of the impact of yoga on breast cancer survivors with aromatase inhibitor-associated arthralgias. Explore: J Sci Heal 8:40–47. doi:10.1016/j.explore.2011.10.002
Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL (2009) Yoga of awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer : Offic J Multinatl Assoc Suppor Care Cancer 17:1301–1309. doi:10.1007/s00520-009-0587-5
Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Palesh OG, Chandwani K, Reddy PS, Melnik MK, Heckler C, Morrow GR (2013) Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. doi:10.1200/JCO.2012.43.7707
American College of Sports M (2010) ACSM’s Guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, Baltimore
Acknowledgments
We thank Susan Rosenthal, MD, for her support on this project. We extend special thanks to the cancer survivors, yoga instructors, and research staff in the University of Rochester Community Clinical Oncology Program (CCOP) Research Base and at each of the National Cancer Institute (NCI) CCOP affiliates who recruited and followed participants in this study. We thank the NCI and the Office of Cancer Complementary and Alternative Medicine for their funding support of this project.
Conflicts of interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peppone, L.J., Janelsins, M.C., Kamen, C. et al. The effect of YOCAS©® yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy. Breast Cancer Res Treat 150, 597–604 (2015). https://doi.org/10.1007/s10549-015-3351-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3351-1