Abstract
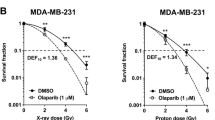
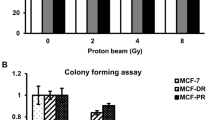
Sustained locoregional control of breast cancer is a significant issue for certain patients. Inhibition of PARP1 is a promising strategy for radiosensitization (RS). We sought to optimize therapy with PARP1 inhibition and radiation (RT) by establishing the most effective treatment schedule, degree of PARP1-mediated RS, and identify early biomarkers predictive of efficacy in breast cancer models. Using clonogenic survival assays, we assessed intrinsic radiosensitivity and RS induced by PARP1 inhibition in breast cancer cell lines. Potential biomarkers of response were evaluated using western blotting, flow cytometry, and immunofluorescence with validation in vivo using tumor xenograft experiments. Across a panel of BC and normal breast epithelial cell lines, the PARP1 inhibitor ABT-888 preferentially radiosensitizes breast cancer (vs. normal) cells with enhancement ratios (EnhR) up to 2.3 independent of intrinsic BC subtype or BRCA mutational status. Concurrent and adjuvant therapy resulted in the highest EnhR of all schedules tested. The degree of RS did not correlate with pretreatment markers of PARP1 activity, DNA damage/repair, or cell cycle distribution. Increases in PARP1 activity 24 h after RT were associated with sensitivity after combination treatment. Findings were confirmed in breast cancer xenograft models. Our study demonstrates that PARP1 inhibition improves the therapeutic index of RT independent of BC subtype or BRCA1 mutational status and that PARP1 activity may serve as a clinically relevant biomarker of response. These studies have led to a clinical trial (TBCRC024) incorporating intratreatment biomarker analyses of PARP1 inhibitors and RT in breast cancer patients.





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Farmer H et al (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434(7035):917–921
Ashworth A (2008) A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 26(22):3785–3790
Turner N, Tutt A, Ashworth A (2004) Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 4(10):814–819
Dantzer F et al (2000) Base excision repair is impaired in mammalian cells lacking poly(ADP-ribose) polymerase-1. Biochemistry 39(25):7559–7569
Mitchell J, Smith GC, Curtin NJ (2009) Poly(ADP-ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys 75(5):1520–1527
Schiewer MJ et al (2012) Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2(12):1134–1149
Weaver AN, Yang ES (2013) Beyond DNA repair: additional functions of PARP-1 in cancer. Front Oncol 3:290
Haber JE (1999) DNA recombination: the replication connection. Trends Biochem Sci 24(7):271–275
Albert JM et al (2007) Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res 13(10):3033–3042
Chalmers A et al (2004) PARP-1, PARP-2, and the cellular response to low doses of ionizing radiation. Int J Radiat Oncol Biol Phys 58(2):410–419
Powell C et al (2010) Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers. Cancer Treat Rev 36(7):566–575
Donawho CK et al (2007) ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 13(9):2728–2737
Rehman S, Reddy CA, Tendulkar RD (2012) Modern outcomes of inflammatory breast cancer. Int J Radiat Oncol Biol Phys 84(3):619–624
Buchholz TA et al (2002) Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol 20(1):17–23
Mamounas EP et al (2012) Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 30(32):3960–3966
Foundation for the National Institutes of Health (2009) I-SPY 2 TRIAL: neoadjuvant and personalized adaptive novel agents to treat breast cancer. ClinicalTrials.gov Identifier: NCT01042379. http://www.clinicaltrials.gov. Accessed 13 Oct 2013
Cancer Research UK (2013) Rucaparib(CO-338;Formally Called AG-014699 or PF-0136738) in treating patients with locally advanced or metastatic breast cancer or advanced ovarian cancer. ClinicalTrials.gov identifier: NCT00664781. http://www.clinicaltrials.gov. Accessed 13 Oct 2013
Gelmon KA et al (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12(9):852–861
Lee J, Annunziata AC, Minasian LM, Zujewski J, Prindiville SA, Kotz HL, Squires J, Houston ND, Ji JJ, Yu M, Doroshow JH, Kohn EC (2011) Phase I study of the PARP inhibitor olaparib (O) in combination with carboplatin (C) in BRCA1/2 mutation carriers with breast (Br) or ovarian (Ov) cancer (Ca) [abstract]. J Clin Oncol 29(Suppl 15):2520
Viswanathan S, Wesolowski W, Layman RM, Alejandra G, Miller B, Chalmers JJ, Bejastani S, Zhao W, Pierluigu G, Cotrill J, Phelps MA, Schaaf LJ, Geyer SM, Hall N, Knopp MV, Shapiro CL, Villalona-Calero MA, Chen A, Grever, Ramaswamy B (2011) A phase I dose-escalation study of ABT-888 (veliparib) in combination with carboplatin in HER2-negative metastatic breast cancer (MBC). J Clin Oncol 29(15):TPS106
Yap TA, Omlin A, de Bono JS (2013) Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol 31(12):1592–1605
de Bono JS, Ashworth A (2010) Translating cancer research into targeted therapeutics. Nature 467(7315):543–549
Horstmann E et al (2005) Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med 352(9):895–904
Tsimberidou AM et al (2012) Personalized medicine in a phase I clinical trials program: the MD Anderson cancer center initiative. Clin Cancer Res 18(22):6373–6383
Baselga J et al (1996) Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 14(3):737–744
O’Brien SG et al (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348(11):994–1004
Flaherty KT et al (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363(9):809–819
Kwak EL et al (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363(18):1693–1703
Ji J, Lee MP, Kadota M, Zhang Y, Parchment RE, Tomaszewski JE, Doroshow JH (2011) Pharmacodynamic and pathway analysis of three presumed inhibitors of poly (ADP-ribose) polymerase: ABT-888, AZD2281, and BSI201 [abstract]. In: Proceedings of the AACR 102nd annual meeting, vol 52, 2011
Han S et al (2013) Targeted radiosensitization of ETS fusion-positive prostate cancer through PARP1 inhibition. Neoplasia 15(10):1207–1217
Fertil B et al (1984) Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res 99(1):73–84
Brenner JC et al (2011) Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell 19(5):664–678
Wei D et al (2013) Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair. Clin Cancer Res 19(16):4422–4432
Neve RM et al (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10(6):515–527
Hollestelle A et al (2010) Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat 121(1):53–64
Elstrodt F et al (2006) BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res 66(1):41–45
Willers H et al (2009) Utility of DNA repair protein foci for the detection of putative BRCA1 pathway defects in breast cancer biopsies. Mol Cancer Res 7(8):1304–1309
Oplustilova L et al (2012) Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment. Cell Cycle 11(20):3837–3850
Barker CA, Powell SN (2010) Enhancing radiotherapy through a greater understanding of homologous recombination. Semin Radiat Oncol 20(4):267–273 e3
Pawlik TM, Keyomarsi K (2004) Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys 59(4):928–942
McCabe N et al (2006) Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 66(16):8109–8115
Veuger SJ et al (2003) Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res 63(18):6008–6015
Hegan DC, Glazer PM (2011) Mechanism of radiosensitization by inhibitors of poly(ADP-ribose) polymerase (PARP) [abstract]. In: Proceedings of the 102nd annual meeting of the american association for cancer research, vol 71, suppl 8, 2011
Ju BG et al (2006) A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312(5781):1798–1802
Zhang F et al (2013) Poly(ADP-ribose) polymerase 1 is a key regulator of estrogen receptor alpha-dependent gene transcription. J Biol Chem 288(16):11348–11357
Riaz M et al (2009) Low-risk susceptibility alleles in 40 human breast cancer cell lines. BMC Cancer 9:236
Hall RE et al (1994) MDA-MB-453, an androgen-responsive human breast carcinoma cell line with high level androgen receptor expression. Eur J Cancer 30A(4):484–490
Poulin R et al (1989) Down-regulation of estrogen receptors by androgens in the ZR-75-1 human breast cancer cell line. Endocrinology 125(1):392–399
Hickey TE et al (2012) Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol 26(8):1252–1267
Saberi A et al (2007) RAD18 and poly(ADP-ribose) polymerase independently suppress the access of nonhomologous end joining to double-strand breaks and facilitate homologous recombination-mediated repair. Mol Cell Biol 27(7):2562–2571
Acknowledgments
The authors would like to thank the Breast Cancer Research Foundation and Komen for the Cure for funding of these studies. We would also like to thank Steven Kronenberg for his excellent assistance in preparing the figures for publication.
Conflict of interest
The authors declare no conflict of interest in this publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Felix Y. Feng and Corey Speers contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, F.Y., Speers, C., Liu, M. et al. Targeted radiosensitization with PARP1 inhibition: optimization of therapy and identification of biomarkers of response in breast cancer. Breast Cancer Res Treat 147, 81–94 (2014). https://doi.org/10.1007/s10549-014-3085-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3085-5