Abstract
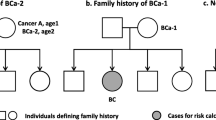
The objective of this nationwide prospective cohort study is to find out the risk of breast cancer (BC) in relatives of patients with multiple BCs by laterality and age at diagnosis of first BC. Having family history of single (HR 1.8; 95 % CI 1.8–1.9) or multiple (HR 2.7; 95 % CI 2.6–2.9) BC was associated with higher risk of BC. Those with an FDR with contralateral BC at any age had the highest risk of familial cancer except at age <40 in which those whose young FDR was affected by multiple ipsilateral BC had the highest risk (HR 9.7; 95 % CI 6.0–15.6). The familial risk of BC in these families decreased as the subject’s and FDRs’ age at diagnosis of first BC increased. The HR was still significantly increased (2.2) for old individuals (>60) having a FDR with contralateral BC at an advanced age (≥80). Despite the common belief that later onset breast cancer is more associated with sporadic breast cancer, our data suggest that breast cancer at any age in the family is associated with some increase in the familial risk, though that risk decreases as the age of onset increases. Contralateral and multiple ipsilateral breast cancers might be associated with distinct shared familial risk factors. Our results have implication for genetic counseling and urge gene identification studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Evans DG, Lalloo F (2002) Risk assessment and management of high risk familial breast cancer. J Med Genet 39(12):865–871
Kharazmi E, Fallah M, Sundquist K, Hemminki K (2012) Familial risk of early and late onset cancer: nationwide prospective cohort study. BMJ 345:e8076
Chang ET, Smedby KE, Hjalgrim H, Glimelius B, Adami HO (2006) Reliability of self-reported family history of cancer in a large case-control study of lymphoma. J Natl Cancer Inst 98(1):61–68
Hampel H, Sweet K, Westman JA, Offit K, Eng C (2004) Referral for cancer genetics consultation: a review and compilation of risk assessment criteria. J Med Genet 41(2):81–91
Hemminki K, Li X, Plna K, Granstrom C, Vaittinen P (2001) The nation-wide Swedish Family Cancer Database: updated structure and familial rates. Acta Oncol 40(6):772–777
The National Board of Health and Welfare CfE (2007) Cancer incidence in Sweden 2006
Horn-Ross PL (1993) Multiple primary cancers involving the breast. Epidemiol Rev 15(1):169–176
Vaittinen P, Hemminki K (2000) Risk factors and age-incidence relationships for contralateral breast cancer. Int J Cancer 88(6):998–1002
Dong C, Hemminki K (2001) Multiple primary cancers of the colon, breast and skin (melanoma) as models for polygenic cancers. Int J Cancer 92(6):883–887
Dong C, Hemminki K (2001) Second primary neoplasms in 633,964 cancer patients in Sweden, 1958–1996. Int J Cancer 93(2):155–161
Sawyer S, Mitchell G, McKinley J, Chenevix-Trench G, Beesley J, Chen XQ, Bowtell D, Trainer AH, Harris M, Lindeman GJ et al (2012) A role for common genomic variants in the assessment of familial breast cancer. J Clin Oncol 30(35):4330–4336
Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, Xue S, Teraoka S, Bernstein L, Capanu M et al (2010) Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol 28(14):2404–2410
Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, Froster UG, Schlehe B, Bechtold A, Arnold N et al (2009) Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 27(35):5887–5892
Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, Olopade OI, Eisen A, Weber B, McLennan J et al (2004) Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 22(12):2328–2335
Reiner AS, John EM, Brooks JD, Lynch CF, Bernstein L, Mellemkjaer L, Malone KE, Knight JA, Capanu M, Teraoka SN et al (2013) Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women’s Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol 31(4):433–439
Chen Y, Thompson W, Semenciw R, Mao Y (1999) Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev 8(10):855–861
Beckmann KR, Buckingham J, Craft P, Dahlstrom JE, Zhang Y, Roder D, Stuart-Harris R (2011) Clinical characteristics and outcomes of bilateral breast cancer in an Australian cohort. Breast 20(2):158–164
Bernstein JL, Thompson WD, Risch N, Holford TR (1992) The genetic epidemiology of second primary breast cancer. Am J Epidemiol 136(8):937–948
Hemminki K, Ji J, Forsti A (2007) Risks for familial and contralateral breast cancer interact multiplicatively and cause a high risk. Cancer Res 67(3):868–870
Ji J, Hemminki K (2007) Risk for contralateral breast cancers in a population covered by mammography: effects of family history, age at diagnosis and histology. Breast Cancer Res Treat 105(2):229–236
McPherson K, Steel CM, Dixon JM (2000) ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ 321(7261):624–628
Johnson N, Fletcher O, Naceur-Lombardelli C, dos Santos SI, Ashworth A, Peto J (2005) Interaction between CHEK2*1100delC and other low-penetrance breast-cancer susceptibility genes: a familial study. Lancet 366(9496):1554–1557
Hartman M, Czene K, Reilly M, Bergh J, Lagiou P, Trichopoulos D, Adami HO, Hall P (2005) Genetic implications of bilateral breast cancer: a population based cohort study. Lancet Oncol 6(6):377–382
Metcalfe K, Gershman S, Lynch HT, Ghadirian P, Tung N, Kim-Sing C, Olopade OI, Domchek S, McLennan J, Eisen A et al (2011) Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 104(9):1384–1392
Hemminki K, Mutanen P (2001) Genetic epidemiology of multistage carcinogenesis. Mutat Res 473(1):11–21
Li CI, Malone KE, Weiss NS, Daling JR (2001) Tamoxifen therapy for primary breast cancer and risk of contralateral breast cancer. J Natl Cancer Inst 93(13):1008–1013
Servant N, Bollet MA, Halfwerk H, Bleakley K, Kreike B, Jacob L, Sie D, Kerkhoven RM, Hupe P, Hadhri R et al (2012) Search for a gene expression signature of breast cancer local recurrence in young women. Clin Cancer Res 18(6):1704–1715
Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81(24):1879–1886
Breast cancer and hormonal contraceptives: further results. Collaborative Group on Hormonal Factors in Breast Cancer. Contraception 54(3 Suppl):1S–106S; 1996
Turkoz FP, Solak M, Petekkaya I, Keskin O, Kertmen N, Sarici F, Arik Z, Babacan T, Ozisik Y, Altundag K (2013) Association between common risk factors and molecular subtypes in breast cancer patients. Breast 22(3):344–350
Thompson D, Easton D (2004) The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia 9(3):221–236
Leu M, Reilly M, Czene K (2008) Evaluation of bias in familial risk estimates: a study of common cancers using Swedish population-based registers. J Natl Cancer Inst 100(18):1318–1325
Barlow L, Westergren K, Holmberg L, Talback M (2009) The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 48(1):27–33
Hemminki K, Mutanen P (2001) Birth order, family size, and the risk of cancer in young and middle-aged adults. Br J Cancer 84(11):1466–1471
Acknowledgments
This work was supported by the Swedish Council for Working Life and Social Research and the German Cancer Aid (Deutsche Krebshilfe).
Conflict of interest
None of the authors declared any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kharazmi, E., Chen, T., Narod, S. et al. Effect of multiplicity, laterality, and age at onset of breast cancer on familial risk of breast cancer: a nationwide prospective cohort study. Breast Cancer Res Treat 144, 185–192 (2014). https://doi.org/10.1007/s10549-014-2848-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2848-3
Keywords
Profiles
- Tianhui Chen View author profile