Abstract
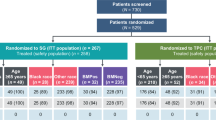
Patients with metastatic triple-negative breast cancer (TNBC) typically have a poor prognosis and limited treatment options. To determine the impact of combining bevacizumab with second-line chemotherapy in patients with metastatic TNBC, we performed an exploratory subgroup analysis of the randomized phase 3 RIBBON-2 trial. RIBBON-2 enrolled patients with metastatic breast cancer that had progressed on first-line non-bevacizumab-containing chemotherapy. After selection of chemotherapy (taxane, gemcitabine, capecitabine, or vinorelbine), patients were randomized 2:1 to receive chemotherapy with either bevacizumab (10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks) or placebo. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate (ORR), and safety. Of 684 patients treated in RIBBON-2, 159 (23%) had TNBC. Baseline characteristics were reasonably balanced in the two treatment groups. The majority received taxane chemotherapy. The hazard ratio (HR) for PFS was 0.494 [95% confidence interval (CI) 0.33–0.74; P = 0.0006]. Median PFS was 6.0 months with bevacizumab–chemotherapy versus 2.7 months with chemotherapy alone. Median OS was 17.9 versus 12.6 months, respectively (HR 0.624, 95% CI 0.39–1.007; P = 0.0534). ORR was 41 versus 18%, respectively (P = 0.0078). The safety profile was consistent with the overall study population and previous phase 3 trials of bevacizumab. Patients with metastatic TNBC derived significant PFS and response benefits from the combination of bevacizumab with second-line chemotherapy. Despite the small sample size and immature data, there was a trend toward improved OS.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Gluz O, Liedtke C, Gottschalk N et al (2009) Triple-negative breast cancer—current status and future directions. Ann Oncol 20:1913–1927
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt 1):4429–4434
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767
O’Shaughnessy J, Osborne C, Pippen JE et al (2011) Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364:205–214
O’Shaughnessy J, Schwartzberg LS, Danso MA et al. (2011) A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J Clin Oncol 29(Suppl): Abstract 1007
O’Shaughnessy J, Dieras V, Glaspy J et al. (2009) Comparison of subgroup analyses of PFS from three phase III studies of bevacizumab in combination with chemotherapy in patients with HER2-negative metastatic breast cancer (MBC). Cancer Res 69(Suppl):512s (Abstract 207)
Gray R, Bhattacharya S, Bowden C et al (2009) Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol 27:4966–4972
Miles DW, Chan A, Dirix LY et al (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28:3239–3247
Robert N, Dieras V, Glaspy G et al (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of HER2-negative locally-recurrent or metastatic breast cancer. J Clin Oncol 29:1252–1260
Brufsky AM, Hurvitz S, Perez E et al (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29:4286–4293
Greenberg S, Rugo H (2010) Triple-negative breast cancer: role of antiangiogenic agents. Cancer J 16:33–38
Rydén L, Jirström K, Haglund M, Stål O, Fernö M (2010) Epidermal growth factor receptor and vascular endothelial growth factor receptor 2 are specific biomarkers in triple-negative breast cancer. Results from a controlled randomized trial with long-term follow-up. Breast Cancer Res Treat 120:491–498
Smith IE, Pierga J-Y, Biganzoli L et al (2011) Final overall survival results and effect of prolonged (≥1 year) first-line bevacizumab-containing therapy for metastatic breast cancer in the ATHENA trial. Breast Cancer Res Treat 130:133–143
Miller KD, Chap LI, Holmes FA et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Baselga J, Stemmer SM, Pego A et al. (2010) Cetuximab + cisplatin in estrogen receptor-negative, progesterone receptor-negative, HER2-negative (triple-negative) metastatic breast cancer: results of the randomized phase II BALI-1 trial. Cancer Res 70(24 Suppl):95s (Abstract PD01–01)
Awada A, Bondarenko IN, Tarasova O et al. (2010) Results of the first randomized phase II study of cationic liposomal paclitaxel (EndoTAG™-1) targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann Oncol 21(Suppl 8):viii5 (Abstract LBA12)
Roché HH, Sparano J, Valero V et al. (2010) Ixabepilone (IXA) and capecitabine (C) in patients (pts) with triple-negative breast cancer (TNBC): a retrospective analysis of phase 2 and phase 3 clinical studies. Ann Oncol 21(Suppl 8):viii103 (Abstract 299P)
Irvin WY, Carey LA (2008) What is triple-negative breast cancer? Eur J Cancer 44:2799–2805
Prat A, Parker JS, Karginova O et al (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12:R68
Acknowledgments
The RIBBON-2 trial was sponsored and funded by Genentech Inc., South San Francisco, CA, USA. The sponsor contributed to the study design and performed the statistical analyses. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Conflict of interest
A. Brufsky has a Consultant/advisory role with Genentech. V. Valero has received research funding and honoraria from Genentech/Roche. H.S. Rugo’s institution has received research funding from Genentech/Roche and Lilly/Imclone. A. Duenne is an employee of F. Hoffmann-La Roche Ltd. N. Bousfoul was formerly a contractor for F. Hoffmann-La Roche Ltd. The other authors have no conflict of interest to declare.
Ethical standards
RIBBON-2 was conducted in accordance with US Food and Drug Administration Good Clinical Practice, International Conference on Harmonisation E6 Guideline for Good Clinical Practice, and national and local ethical and legal requirements. All patients provided written informed consent before study-specific screening.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brufsky, A., Valero, V., Tiangco, B. et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat 133, 1067–1075 (2012). https://doi.org/10.1007/s10549-012-2008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2008-6
Keywords
Profiles
- Adam Brufsky View author profile