Abstract
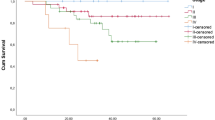
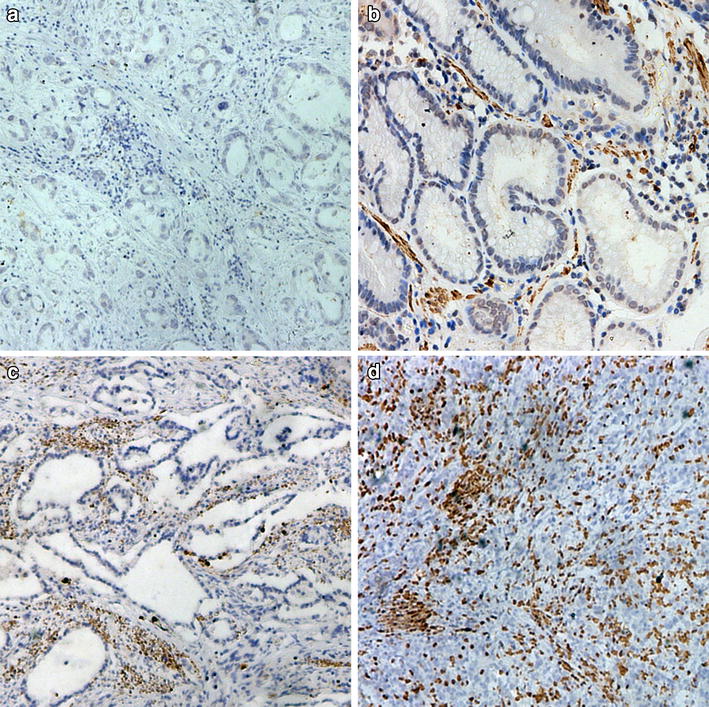
Purpose Patients with locally advanced breast cancer (LABC) have a poor outcome. A molecular predictor to identify at-risk patients is sorely needed. CXCR4 is a chemokine receptor that has been linked to breast cancer invasion and metastasis. We postulate that in patients with LABC, CXCR4 overexpression levels in cancer specimens following neoadjuvant chemotherapy predict cancer outcome. Experimental design 54 patients with LABC were prospectively accrued and analyzed. All had neoadjuvant chemotherapy and definitive surgical therapy. Study homogeneity was maintained by standardized treatment, surveillance, and compliance protocols. A 1 cm3 cancer from the surgical specimens of each patient was retrieved for analysis. CXCR4 levels were detected using Western blots, and results were quantified against 1 μg of protein from HeLa cells. CXCR4 expression was defined as low (<6.6-fold) or high (≥6.6-fold). Primary endpoints were cancer recurrence and death. Statistical analysis performed included independent samples t-test, chi-square test, Spearman Rank analysis, Kaplan-Meier survival analysis, log-rank test, and Cox proportional hazard model. Results With a median follow-up of 30 months, patients with high CXCR4 overexpression (≥6.6-fold) had a significantly higher incidence of recurrence (P = 0.0006) and cancer death (P = 0.0128) than those with low CXCR4 overexpression (<6.6-fold). The relative risks for recurrence and death in the high CXCR4 group were 27.3-fold (95% CI: 6.2–120.8; P = 0.001) and 4.8-fold (95% CI: 1.5–15.0; P = 0.0076) higher, respectively than those in the low CXCR4 group. Conclusion High CXCR4 overexpression in specimens from LABC patients receiving neoadjuvant chemotherapy was predictive of cancer outcome.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Hortobagyi GN (1990) Comprehensive management of locally advanced breast cancer. Cancer 66:1387–1391
Hortobagyi GN, Ames FC, Buzdar AU, Kau SW, McNeese MD, Paulus D, Hug V, Holmes FA, Romsdahl MM, Fraschini G et al (1988) Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 62:2507–2516
Fisher B, Gunduz N, Saffer EA (1983) Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res 43:1488–1492
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women. with operable breast cancer. J Clin Oncol 16:2672–2685
Goble S, Bear HD (2003) Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: the potential and the questions. Surg Clin North Am 83:943–971
Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Elledge R, Mohsin S, Osborne CK, Chamness GC, Allred DC, O’Connell P (2003) Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 362:362–369
Scholl SM, Pierga JY, Asselain B, Beuzeboc P, Dorval T, Garcia-Giralt E, Jouve M, Palangie T, Remvikos Y, Durand JC (1995) Breast tumour response to primary chemotherapy predicts local and distant control as well as survival. Eur J Cancer 31A(12):1969–1975
Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK, Orlov AA, Barash NY, Golubeva OM, Chepic OF (1994) Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb–IIIa breast cancer. Ann Oncol 5(7):591–595
Makris A, Powles TJ, Dowsett M, Osborne CK, Trott PA, Fernando IN, Ashley SE, Ormerod MG, Titley JC, Gregory RK, Allred DC (1997) Prediction of response to neoadjuvant chemoendocrine therapy in primary breast carcinomas. Clin Cancer Res 3(4):593–600
Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, Dilhuydy JM, Bonichon F (1999) Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 10(1):47–52
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19(22):4224–4237
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17(2):460–469
Pierga JY, Mouret E, Dieras V, Laurence V, Beuzeboc P, Dorval T, Palangie T, Jouve M, Vincent-Salomon A, Scholl S, Extra JM, Asselain B, Pouillart P (2000) Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br J Cancer 83(11):1480–1487
McCready DR, Hortobagyi GN, Kau SW et al (1989) The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg 124:21–25
Botti C, Vici P, Lopez M et al (1995) Prognostic value of lymph node metastases after neoadjuvant chemotherapy for large-sized operable carcinoma of the breast. J Am Coll Surg 181:202–208
Kuerer HM, Newman LA, Buzdar AU et al (1998) Residual metastatic ALNs following neoadjuvant chemotherapy predicts disease-free survival in locally advanced breast cancer patients. Am J Surg 176:502–509
Cance WG, Carey LA, Calvo BF et al (2002) Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg 236:295–303
Carey LA, Metzger R, Dees EC et al (2005) American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst 97:1137–1142
Müller A, Homey B, Soto H, Ge N et al (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56
Holm N, Byrnes K, Li B, Turnage R, Abreo F, Mathis J, Chu QD (2007) Elevated levels of chemokine receptor CXCR4 in HER-2 negative breast cancer specimens predict recurrence. J Surg Res 141:53–59
Lee B, Lee T, Avraham S, Avraham H (2004) Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mole Cancer Res 2:327–338
Liang Z, Yoon Y, Votaw J et al (2005) Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res 65:967–971
Lapteva N, Yang A, Sanders D et al (2005) CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther 12:84–89
Smith M, Luker K, Garbow J et al (2004) CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 64:8604–8612
American Joint Committee on Cancer (2002) AJCC Cancer Staging Manual, 6th edn
Tiezzi D, Andrade J, Ribeiro-Silva A, Zola F et al (2007) HER-2, p53, p21 and hormonal receptors proteins expression as predictive factors of response and prognosis in locally advanced breast cancer treated with neoadjuvant docetaxel plus epirubicin combination. BMC Cancer 7:36
Singletary S, McNeese M, Hortobagyi G (1992) Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma. Cancer 69:2849–2852
Alassas M, Chu Q, Burton G, Ampil F et al (2005) Neoadjuvant chemotherapy in stage III breast cancer. Am Surgeon 71:487–492
Isaccs C, Stearns V, Hayes DF (2001) New prognostic factors for breast cancer recurrence. Semin Oncol 28:53–67
Gianni L, Zambetti M, Clark K, Baker J et al (2005) Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol 23:7265–7277
Hess K, Anderson K, Symmans W, Valero V et al (2006) Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol 24:4236–4244
Sørlie T, Perou C, Fan C, Geisler S et al (2006) Gene expression profiles do not consistently predict the clinical treatment response in locally advanced breast cancer. Mol Cancer Ther 5:2914–2918
Pierga J, Reis-Filho J, Cleator S, Dexter T et al (2007) Microarray-based comparative genomic hybridisation of breast cancer patients receiving neoadjuvant chemotherapy. Br J Cancer 96:341–351
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Palangie T, Mosseri V, Mihura J, Campana F et al (1994) Prognostic factors in inflammatory breast cancer and therapeutic implications. Eur J Cancer 30A:921–927
Honkoop A, van Diest P, de Jong J et al (1998) Prognostic role of clinical, pathological and biological characteristics in patients with locally advanced breast cancer. Br J Cancer 77:621–626
Proudfoot A (2002) Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol 2:106–115
Li Y, Pan Y, Wei Y, Cheng X et al (2004) Upregulation of CXCR4 is essential for HER-2 mediated tumor metastasis. Cancer Cell 6:459–469
Author information
Authors and Affiliations
Corresponding author
Additional information
Neal T. Holm was the recipient of the American Society of Clinical Oncology 2007 Merit Award
Rights and permissions
About this article
Cite this article
Holm, N.T., Abreo, F., Johnson, L.W. et al. Elevated chemokine receptor CXCR4 expression in primary tumors following neoadjuvant chemotherapy predicts poor outcomes for patients with locally advanced breast cancer (LABC). Breast Cancer Res Treat 113, 293–299 (2009). https://doi.org/10.1007/s10549-008-9921-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-9921-8