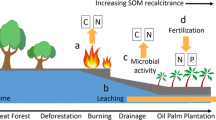
Abstract
Extensive draining at tropical ombrotrophic peatlands in Southeast Asia has made these landscapes a global ‘hot spots’ for greenhouse gas emissions. Management practices and fires have changed substrate status, which affects microbial processes. Here, we present data on how change in management practices affect carbon (C) mineralization processes at these soils. We compared the C mineralization potentials of undrained swamp forest peat to those of abandoned peat (deforested, drained and burned peatlands in degraded condition) at various depths, with and without additional substrates (glucose, glutamate and nitrate), under oxic and anoxic conditions through ex situ experiments. Carbon mineralization (CO2 and CH4 production) rates were higher in the forest peat, with higher litter deposition and C availability. Production rates decreased with peat depth coinciding with decreasing availability of labile C. Consequently, the increase in production rates after labile substrate addition was relatively modest in forest peat as compared to the abandoned site and from the top layers as compared to deeper layers. Methanogenesis had little importance in total C loss. Adding labile C and nitrogen (N) enhanced heterotrophic CO2 production more than only addition of N. Surprisingly, oxygen availability did not limit CO2 production rates, but anoxic respiration also yielded substantial rates, especially at the forest peat. Flooding of these sites will therefore reduce, but not completely cease, peat C-loss. Reintroduced vegetation and fertilization in abandoned peatlands can enrich the peat with labile C and N compounds and thus lead to increased microbiological activity.





Similar content being viewed by others
References
Adji FF, Hamada Y, Darung U, Limin SH, Hatano R (2014) Effect of plant-mediated oxygen supply and drainage on greenhouse gas emission from a tropical peatland in Central Kalimantan, Indonesia. Soil Sci Plant Nutr 60:216–230
Arai H, Hadi A, Darung U, Limin SH, Hatano R, Inubushi K (2014) A methanotrophic community in a tropical peatland is unaffected by drainage and forest fires in a tropical peat soil. Soil Sci Plant Nutr 60:577–585
Benner R, Maccubbin AE, Hodson RE (1984) Anaerobic biodegradation of the lignin and polysaccharide components of lignocellulose and synthetic lignin by sediment microflora. Appl Environ Microb 47:998–1004
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fert Soils 45:115–131
Brady M (1997) Organic matter dynamics of coastal peat deposits in Sumatra. The University of British Columbia, Canada
Corbett JE, Tfaily MM, Burdige DJ, Glaser PH, Chanton JP (2015) The relative importance of methanogenesis in the decomposition of organic matter in northern peatlands. J Geophys Res Biogeosci 120:280–293
DeBusk WF, Reddy KR (1998) Turnover of detrital organic carbon in a nutrient-impacted Everglades marsh. Soil Sci Soc Am Pro 62:1460–1468
Dommain R, Cobb A, Joosten H, Glaser PH, Chua AFL, Gandois L, Kai F-M, Noren A, Salim KA, Su’ut NSH, Harvey CF (2015) Forest dynamics and tip-up pools drive pulses of high carbon accumulation rates in a tropical peat dome in Borneo (Southeast Asia). J Geophys Res Biogeosci 120:617–640
Drösler M, Verchot LV, Freibauer A et al (2014) Drained inland organic soils. In: Hiraishi T, Krug T, Tanabe K, Srivastava N, Baasansuren J, Fukuda M, Troxler TG (eds) Supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: wetlands. IPCC, Switzerland, p 353
Duddleston KN, Kinney MA, Kiene RP, Hines ME (2002) Anaerobic microbial biogeochemistry in a northern bog: acetate as a dominant metabolic end product. Global Biogeochem Cycles 16:1063. doi:10.1029/2001GB001402
Emsens W-J, Aggenbach CJS, Schoutens K, Smolders AJP, Zak D, van Diggelen R (2016) Soil iron content as a predictor of carbon and nutrient mobilization in rewetted fens. PLoS One 11:e0153166. doi:10.1371/journal.pone.0153166
Fahmi A, Radjagukguk B, Purwanto BH, Hanudin E (2010) The role of peat layers on iron dynamics in peatlands. JTS 15:195–201
Funakawa S, Yonebayashi K, Jong FS, Oi Khun EC (1996) Nutritional environment of tropical peat soils in Sarawak, Malaysia based on soil solution composition. Soil Sci Plant Nutr 42:833–843
Galand PE, Yrjälä K, Conrad R (2010) Stable carbon isotope fractionation during methanogenesis in three boreal peatland ecosystems. Biogeosciences 7:3893–3900
GFED (2016) Global fire emissions database, http://www.globalfiredata.org/updates.html. Accessed 22 Feb 2016
Glatzel S, Basiliko N, Moore T (2004) Carbon dioxide and methane production potentials of peats from natural, harvested and restored sites, Eastern Québec, Canada. Wetlands 24:261–267
Hadi A, Haridi M, Inubushi K, Purnomo E, Razie F, Tsuruta H (2001) Effects of land-use change in tropical peat soil on the microbial population and emission of greenhouse gases. Microbes Environ 16:79–86
Harmon ME, Silver WL, Fasth B, Chen H, Burke IC, Parton WJ, Hart SC, Currie WS, Lidet (2009) Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Global Change Biol 15:1320–1338
Heitmann T, Goldhammer T, Beer J, Blodau C (2007) Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Global Change Biol 13:1771–1785
Hirano T, Jauhiainen J, Inoue T, Takahashi H (2009) Controls on the carbon balance of tropical peatlands. Ecosystems 12:873–887
Hirano T, Kusin K, Limin S, Osaki M (2014) Carbon dioxide emissions through oxidative peat decomposition on a burnt tropical peatland. Global Change Biol 20:555–565
Hooijer A, Page S, Canadell JG, Silvius M, Kwadijk J, Wösten H, Jauhiainen J (2010) Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 7:1505–1514
Jauhiainen J, Takahashi H, Heikkinen JEP, Martikainen PJ, Vasander H (2005) Carbon fluxes from a tropical peat swamp forest floor. Global Change Biol 11:1788–1797
Jauhiainen J, Limin S, Silvennoinen H, Vasander H (2008) Carbon dioxide and methane fluxes in drainage affected tropical peat before and after hydrological restoration. Ecology 89:3503–3514
Jauhiainen J, Kerojoki O, Silvennoinen H, Limin S, Vasander H (2014) Heterotrophic respiration in drained tropical peat is greatly affected by temperature—a passive ecosystem cooling experiment. Environ Res Lett 9:105013. doi:10.1088/1748-9326/9/10/105013
Kane ES, Chivers MR, Turetsky MR, Treat CC, Petersen DG, Waldrop M, Harden JW, McGuire AD (2013) Response of anaerobic carbon cycling to water table manipulation in an Alaskan rich fen. Soil Biol Biochem 58:50–60
Könönen M, Jauhiainen J, Laiho R, Kusin K, Vasander H (2015) Tropical peat physical and chemical properties on stabilized land-use types. Mires and peat 16: Article 08, 1–13. http://www.mires-and-peat.net/pages/volumes/map16/map1608.php)
Könönen M, Jauhiainen J, Laiho R, Spetz P, Kusin K, Limin S, Vasander H (2016) Land use increases the recalcitrance of tropical peat. Wetl Ecol Manag. doi:10.1007/s11273-016-9498-7
Kristensen E, Ahmed SI, Devol AH (1995) Aerobic and anaerobic decomposition of organic matter in marine sediment: which is fastest? Limnol Oceanogr 40:1430–1437
Lampela M, Jauhiainen J, Vasander H (2014) Surface peat structure and chemistry in a tropical peat swamp forest. Plant Soil 382:329–347
Lampela M, Jauhiainen J, Kämäri I, Koskinen M, Tanhuanpää T, Valkeapää A, Vasander H (2016) Ground surface microtopography and vegetation patterns in a tropical peat swamp forest. Catena 139:127–136
Langman B, Graf H (2003) Indonesian smoke aerosols from peat fires and the contribution from volcanic sulfur emissions. Geophys Res Lett 30:1547. doi:10.1029/2002GL016646
Lipson DA, Jha M, Raab TK, Oechel WC (2010) Reduction of iron (III) and humic substances plays a major role in anaerobic respiration in an Arctic peat soil. J Geophys Res 115:G00I06. doi:10.1029/2009JG001147
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Miettinen J, Shi C, Liew SC (2016) Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Global Ecol Conservation 6:67–78
Miyajima T, Wada E, Hanba YT, Vijarnsorn P (1997) Anaerobic mineralization of indigenous organic matters and methanogenesis in tropical wetland soils. GCA 61:3739–3751
Molstad L, Dörsh P, Bakken LR (2007) Robotized incubation system for monitoring gases (O2, NO, N2O N2) in denitrifying cultures. J Microbiol Meth 71:202–211
Moore TR, Dalva M (1997) Methane and carbon dioxide exchange potentials of peat soils in aerobic and anaerobic laboratory incubations. Soil Biol Biochem 29:1157–1164
Moore S, Evans CD, Page SE, Garnett MH, Jones TG, Freeman C, Hoojier A, Wiltshire A, Limin SH, Gauci V (2013) Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes. Nature 493:660–663
Morley RJ (1981) Development and vegetation dynamics of a lowland ombrogenous peat swamp in Kalimantan Tengah, Indonesia. J Biogeogr 8:383–404
Nadeem S, Dörsch P, Bakken LR (2013) Autoxidation and acetylene-accelerated oxidation of NO in a 2-phase system: implications for the expression of denitrification in ex situ experiments. Soil Biol Biochem 57:606–614
Page SE, Siegert F, Rieley JO, Boehm HDV, Jaya A, Limin S (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420:61–65
Sundari S, Hirano T, Yamada H, Kusin K, Limin S (2012) Effect of groundwater level on soil respiration in tropical peat swamp forests. J-Stage 68:121–134
Vile MA, Bridgham SD, Wieder RK, Novák M (2003) Atmospheric sulfur deposition alters pathways of gaseous carbon production in peatlands. Global Biogeochem Cycles 17:1058–1064
Weiss D, Shotyk W, Rieley J, Page S, Gloor M, Reese S, Martinez-Cortizas A (2002) The geochemistry of major and selected trace elements in a forested peat bog, Kalimantan, SE Asia, and its implications for past atmospheric dust deposition. CGA 66:2307–2323
Yule C, Comez L (2009) Leaf litter decomposition in a tropical peat swamp forest in Peninsular Malaysia. Wetl Ecol Manag 17:231–241
Yule CM, Lim YY, Lim TY (2016) Degradation of tropical Malaysian peatlands decreases levels of phenolics in soil and in leaves of Macaranga pruinosa. Front Earth Sci 4:45. doi:10.3389/feart.2016.00045
Acknowledgments
We owe special thanks to Ms. Maiju Piirainen, Mr. Javier Andrés Jiménez and Mr. Trygve Fredriksen for their invaluable help in the laboratory. Our study was part of the Academy of Finland-funded project ‘Restoration Impact on Tropical Peat Carbon and Nitrogen Dynamics’ (RETROPEAT). Research permission for the project was granted by the Indonesian Ministry of Research and Technology (RISTEK). The Peatlanders prize money awarded by the University of Helsinki was important for covering some costs incurred by the laboratory work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Charles T. Driscoll.
Rights and permissions
About this article
Cite this article
Jauhiainen, J., Silvennoinen, H., Könönen, M. et al. Management driven changes in carbon mineralization dynamics of tropical peat. Biogeochemistry 129, 115–132 (2016). https://doi.org/10.1007/s10533-016-0222-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-016-0222-8