Abstract
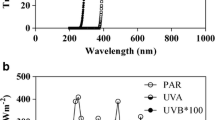
The influence of solar ultraviolet radiation and photosynthetically active radiation (PAR) on summertime marine bacterial uptake and assimilation of sulfur from radiolabeled dimethlysulfoniopropionate (35S-DMSP) was studied at four Arctic and two Antarctic stations. Incubations with 3H-leucine were also conducted for comparative purposes as a measurement of bacterial activity. Arctic waters were characterized by large numbers of colonial Phaeocystis pouchetii and higher DMSP concentrations than in the two diatom-dominated Antarctic samples. Exposure to full sunlight radiation (280–700 nm), and to a lesser extent to PAR + UVA (320–700 nm), generally decreased the bacterial assimilation of 3H-leucine with respect to darkness, and caused variable effects on 35S-DMSP assimilation. By using a single-cell approach involving microautoradiography we found high percentages of sulfur assimilating cells within the bacterial groups Gammaproteobacteria, Bacteroidetes, SAR11 and Roseobacter despite the varying DMSP concentrations between Arctic and Antarctic samples. The dominant SAR11 clade contributed 50–70% of the cells assimilating both substrates in the Arctic stations, whereas either Gammaproteobacteria or SAR11 were the largest contributors to 3H-leucine uptake in samples from the two Antarctic stations. Only one station was analyzed for single-cell 35S-DMSP assimilation in Antarctica, and Gammaproteobacteria were major contributors to its uptake, providing the first evidence for Antarctic bacteria actively taking up 35S-DMSP. PAR + UVA repeatedly increased the number of SAR11 cells assimilating 3H-leucine. This pattern also occurred with other 35S-DMSP assimilating groups, though not so consistently. Our results support a widespread capability of polar bacteria to assimilate DMSP-sulfur during the season of maximum DMSP concentrations, and show for the first time that all major polar taxa can be highly active at this assimilation under the appropriate circumstances. Our findings further confirm the role of sunlight as a modulator of heterotrophic carbon and sulfur fluxes in the surface ocean.







Similar content being viewed by others
References
Aas P, Lyons MM, Pledger R, Mitchell DL, Jeffrey WH (1996) Inhibition of bacterial activities by solar radiation in nearshore waters and the Gulf of Mexico. Aquat Microb Ecol 11:229–238
Alonso C, Pernthaler J (2005) Incorporation of glucose under anoxic conditions by bacterioplankton from coastal North Sea surface waters. Appl Environ Microbiol 71:1709–1716
Alonso C, Pernthaler J (2006) Concentration-dependent patterns of leucine incorporation by coastal picoplankton. Appl Environ Microbiol 72:2141–2147
Alonso-Sáez L, Gasol JM, Lefort T, Hofer J, Sommaruga R (2006) Effect of natural sunlight on bacterial activity and differential sensitivity of natural bacterioplankton groups in northwestern Mediterranean coastal waters. Appl Environ Microbiol 72:5806–5813
Alonso-Sáez L, Sánchez O, Gasol JM, Balagué V, Pedrós-Alió C (2008) Winter-to-summer changes in the composition and single-cell activity of near-surface Arctic prokaryotes. Environ Microbiol 10:2444–2454
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Archer SD, Ragni M, Webster R, Airs RL, Geiderb RJ (2010) Dimethyl sulfoniopropionate and dimethyl sulfide production in response to photoinhibition in Emiliania huxleyi. Limnol Oceanogr 55:1579–1589
Arrieta JM, Weinbauer MG, Herndl GJ (2000) Interspecific variability in sensitivity to UV radiation and subsequent recovery in selected isolates of marine bacteria. Appl Environ Microbiol 66:1468–1473
Bano N, Hollibaugh JT (2002) Phylogenetic composition of bacterioplankton assemblages from the Arctic Ocean. Appl Environ Microbiol 68:505–518
Béjà O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, Jovanovich S, Gates CM, Feldman RA, Spudich JL, Spudich EN, DeLong EF (2000) Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289:1902–1906
Brussaard CPD, Mari X, Van Bleijswijk JDL, Veldhuis MJW (2005) A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics—II. Significance for the microbial community. Harmful Algae 4:875–893
Buma AGJ, Helbling EW, de Boer MK, Villafañe VE (2001) Patterns of DNA damage and photoinhibition in temperate South-Atlantic picophytoplankton exposed to solar ultraviolet radiation. J Photoch Phobio B 62:9–18
Burkill PH, Archer SD, Robinson C, Nightingale PD, Groom SB, Tarran GA, Zubkov MV (2002) Dimethyl sulphide biogeochemistry within a coccolithophore bloom (DISCO): an overview. Deep Sea Res Pt II 49:2863–2885
Church MJ, Ducklow HW, Karl DA (2004) Light dependence of [H-3]leucine incorporation in the oligotrophic North Pacific ocean. Appl Environ Microbiol 70:4079–4087
Convey P, Fogg GE (2007) The effects of radiation. In: Thomas DN, Fogg GE, Convey P, Fritsen CH, Gili JM, Gradinger R, Laybourn-Parry J, Reid K, Walton DWH (eds) The biology of polar regions. Oxford University, New York, pp 42–49
Cottrell MT, Kirchman DL (2000) Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66:1692–1697
Cottrell MT, Kirchman DL (2009) Photoheterotrophic microbes in the Arctic Ocean in summer and winter. Appl Environ Microbiol 75:4958–4966
Curran MAJ, Jones GB (2000) Dimethyl sulfide in the Southern Ocean: seasonality and flux. J Geophys Res 105:20451–20459
Daims H, Bruhl A, Amann R, Schleifer KH, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
Davidson AT, van der Heijden A (2000) Exposure of natural Antarctic marine microbial assemblages to ambient UV radiation: effects on bacterioplankton. Aquat Microb Ecol 21:257–264
DeLong EF, Franks DG, Alldredge AL (1993) Phylogenetic diversity of aggregate-attached vs free-living marine bacterial assemblages. Limnol Oceanogr 38:924–934
Eilers H, Pernthaler J, Peplies J, Glockner FO, Gerdts G, Amann R (2001) Isolation of novel pelagic bacteria from the German bight and their seasonal contributions to surface picoplankton. Appl Environ Microbiol 67:5134–5142
Elifantz H, Malmstrom RR, Cottrell MT, Kirchman DL (2005) Assimilation of polysaccharides and glucose by major bacterial groups in the Delaware Estuary. Appl Environ Microbiol 71:7799–7805
Elifantz H, Dittell AI, Cottrell MT, Kirchman DL (2007) Dissolved organic matter assimilation by heterotrophic bacterial groups in the western Arctic Ocean. Aquat Microb Ecol 50:39–49
Fogg GE (1977) Aquatic primary production in the Antarctic. Philos Trans R Soc Lond B Biol Sci 279:27–38
Gasol JM, Del Giorgio PA (2000) Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64:197–224
Gentile G, Giuliano L, D’Auria G, Smedile F, Azzaro M, De Domenico M, Yakimov MM (2006) Study of bacterial communities in Antarctic coastal waters by a combination of 16S rRNA and 16S rDNA sequencing. Environ Microbiol 8:2150–2161
Gómez-Consarnau L, González JM, Coll-Lladó M, Gourdon P, Pascher T, Neutze R, Pedrós-Alió C, Pinhassi J (2007) Light stimulates growth of proteorhodopsin-containing marine Flavobacteria. Nature 445:210–213
González JM, Kiene RP, Moran MA (1999) Transformation of sulfur compounds by an abundant lineage of marine bacteria in the alpha-subclass of the class Proteobacteria. Appl Environ Microbiol 65:3810–3819
González JM, Simó R, Massana R, Covert JS, Casamayor EO, Pedrós-Alió C, Moran MA (2000) Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl Environ Microbiol 66:4237–4246
Hernández EA, Ferreyra GA, Mac Cormack WP (2006) Response of two Antarctic marine bacteria to different natural UV radiation doses and wavelengths. Antarct Sci 18:205–212
Herndl GJ, Müller-Niklas G, Frick J (1993) Major role of ultraviolet B in controlling bacterioplankton growth in the surface layer of the ocean. Nature 361:717–719
Herndl GJ, Brugger A, Hager S, Kaiser E, Obernosterer I, Reitner B, Slezak D (1997) Role of ultraviolet-B radiation on bacterioplankton and the availability of dissolved organic matter. Plant Ecol 128:42–51
Jones AE, Shanklin JD (1995) Continued decline of ozone over Halley, Antarctica, since 1985. Nature 376:409–411
Kaiser E, Herndl GJ (1997) Rapid recovery of marine bacterioplankton activity after inhibition by UV radiation in coastal waters. Appl Environ Microbiol 63:4026–4031
Karsten UK, Kuck K, Vogt C, Kirst GO (1996) Dimethylsulfoniopropionate production in phototrophic organisms and its physiological function as cryoprotectant. In: Kiene RP, Visscher PT, Keller MD, Kirst GO (eds) Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, pp 143–153
Keller MD, Bellows WK, Guillard RRL (1989) Dimethyl sulfide production in marine phytoplankton. In: Saltzman E, Cooper WJ (eds) Biogenic sulfur in the environment. American Chemical Society, New York, pp 167–182
Kiene RP, Slezak D (2006) Low dissolved DMSP concentrations in seawater revealed by small-volume gravity filtration and dialysis sampling. Limnol Oceanogr-Meth 4:80–95
Kiene RP, Linn LJ, Gonzalez J, Moran MA, Bruton JA (1999) Dimethylsulfoniopropionate and methanethiol are important precursors of methionine and protein-sulfur in marine bacterioplankton. Appl Environ Microbiol 65:4549–4558
Kiene RP, Linn LJ, Bruton JA (2000) New and important roles for DMSP in marine microbial communities. J Sea Res 43:209–224
Kirchman D, Knees E, Hodson R (1985) Leucine incorporation and its potential as a measure of protein-synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol 49:599–607
Kirst GO (1996) Osmotic adjustment in phytoplankton and macroalgae: the use of dimethylsulfoniopropionate (DMSP). In: Kiene RP, Visscher PT, Keller MD, Kirst GO (eds) Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum, New York, pp 121–129
Kolber ZS, Van Dover CL, Niederman RA, Falkowski PG (2000) Bacterial photosynthesis in surface waters of the open ocean. Nature 407:177–179
Lasternas S, Agustí S (2010) Phytoplankton community structure during the record Arctic ice-melting of summer 2007. Polar Biol 33:1709–1717
Liss PS, Malin G, Turner SM, Holligan PM (1994) Dimethyl sulfide and Phaeocystis: a review. J Marine Syst 5:41–53
Malmstrom RR, Kiene RP, Cottrell MT, Kirchman DL (2004a) Contribution of SAR11 bacteria to dissolved dimethylsulfoniopropionate and amino acid uptake in the North Atlantic Ocean. Appl Environ Microbiol 70:4129–4135
Malmstrom RR, Kiene RP, Kirchman DL (2004b) Identification and enumeration of bacteria assimilating dimethylsulfoniopropionate (DMSP) in the North Atlantic and Gulf of Mexico. Limnol Oceanogr 49:597–606
Malmstrom RR, Straza TRA, Cottrell MT, Kirchman DL (2007) Diversity, abundance, and biomass production of bacterial groups in the western Arctic Ocean. Aquat Microb Ecol 47:45–55
Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH (1992) Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst Appl Microbiol 15:593–600
Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer H (1996) Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga–Flavobacter–Bacteroides in the natural environment. Microbiology 142:1097–1106
Matrai P, Vernet M (1997) Dynamics of the vernal bloom in the marginal ice-zone of the Barents Sea: DMS and DMSP budgets. J Geophys Res 102:22965–22979
Morris RM, Rappe MS, Connon SA, Vergin KL, Siebold WA, Carlson CA, Giovannoni SJ (2002) SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420:806–810
Müller R, Crutzen PJ, Grooss JU, Bruhl C, Russell JM, Gernandt H, McKenna DS, Tuck AF (1997) Severe chemical ozone loss in the Arctic during the winter of 1995–96. Nature 389:709–712
Murray AE, Wu KY, Moyer CL, Karl DM, DeLong EF (1999) Evidence for circumpolar distribution of planktonic Archaea in the Southern Ocean. Aquat Microb Ecol 18:263–273
Neale PJ, Cullen JJ, Davis RF (1998) Inhibition of marine photosynthesis by ultraviolet radiation: variable sensitivity of phytoplankton in the Weddell-Scotia confluence during the austral spring. Limnol Oceanogr 43:433–448
Pakulski JD, Kase JP, Meador JA, Jeffrey WH (2008) Effect of stratospheric ozone depletion and enhanced ultraviolet radiation on marine bacteria at Palmer Station, Antarctica in the early austral spring. Photochem Photobiol 84:215–221
Pernthaler A, Pernthaler J, Amann R (2002) Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol 68:3094–3101
Pinhassi J, Sala MM, Havskum H, Peters F, Guadayol O, Malits A, Marrase CL (2004) Changes in bacterioplankton composition under different phytoplankton regimens. Appl Environ Microbiol 70:6753–6766
Pinhassi J, Simó R, González JM, Vila M, Alonso-Sáez L, Kiene RP, Moran MA, Pedrós-Alió C (2005) Dimethylsulfoniopropionate turnover is linked to the composition and dynamics of the bacterioplankton assemblage during a microcosm phytoplankton bloom. Appl Environ Microbiol 71:7650–7660
Rich J, Gosselin M, Sherr E, Sherr B, Kirchman DL (1997) High bacterial production, uptake and concentrations of dissolved organic matter in the Central Arctic Ocean. Deep Sea Res Pt II 44:1645–1663
Riemann L, Steward GF, Azam F (2000) Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl Environ Microbiol 66:578–587
Rignot E, Bamber JL, Van Den Broeke MR, Davis C, Li YH, Van De Berg WJ, Van Meijgaard E (2008) Recent Antarctic ice mass loss from radar interferometry and regional climate modelling. Nat Geosci 1:106–110
Rothrock DA, Yu Y, Maykut GA (1999) Thinning of the Arctic sea-ice cover. Geophys Res Lett 26:3469–3472
Ruiz-González C, Galí M, Sintes E, Herndl GJ, Gasol JM, Simó R Sunlight effects on the osmoheterotrophic behaviour of Arctic and Antarctic phytoplankton. Environ Microbiol (Submitted)
Ruiz-González C, Lefort T, Galí M, Sala MM, Sommaruga R, Simó R, Gasol JM (2012) Seasonal patterns in the sunlight sensitivity of bacterioplankton from Mediterranean surface coastal waters. FEMS Microbiol Ecol. doi:10.1111/j.1574-6941.2011.01247.x
Sakka A, Gosselin M, Levasseur M, Michaud S, Monfort P, Demers S (1997) Effects of reduced ultraviolet radiation on aqueous concentrations of dimethylsulfoniopropionate and dimethylsulfide during a microcosm study in the Lower St. Lawrence Estuary. Mar Ecol Prog Ser 149:227–238
Sakshaug E (2004) Primary and secondary production in the Arctic Seas. In: Stein R, Macdonald RW (eds) The organic carbon cycle in the Arctic Ocean. Springer, New York, pp 57–81
Saló V, Simó R, Vila-Costa M, Calbet A (2009) Sulfur assimilation by Oxyrrhis marina feeding on a 35S-DMSP-labelled prey. Environ Microbiol 11:3063–3072
Simó R (2001) Production of atmospheric sulfur by oceanic plankton: biogeochemical, ecological and evolutionary links. Trends Ecol Evol 16:287–294
Simó R, Grimalt JO, Albaiges J (1996) Sequential method for the field determination of nanomolar concentrations of dimethyl sulfoxide in natural waters. Anal Chem 68:1493–1498
Simó R, Archer SD, Pedrós-Alió C, Gilpin L, Stelfox-Widdicombe CE (2002) Coupled dynamics of dimethylsulfoniopropionate and dimethylsulfide cycling and the microbial food web in surface waters of the North Atlantic. Limnol Oceanogr 47:53–61
Simó R, Vila-Costa M, Alonso-Sáez L, Cardelús C, Guadayol O, Vázquez-Domínguez E, Gasol JM (2009) Annual DMSP contribution to S and C fluxes through phytoplankton and bacterioplankton in a NW Mediterranean coastal site. Aquat Microb Ecol 57:43–55
Simon M, Glockner FO, Amann R (1999) Different community structure and temperature optima of heterotrophic picoplankton in various regions of the Southern Ocean. Aquat Microb Ecol 18:275–284
Sintes E, Herndl GJ (2006) Quantifying substrate uptake by individual cells of marine bacterioplankton by catalyzed reporter deposition fluorescence in situ hybridization combined with micro autoradiography. Appl Environ Microbiol 72:7022–7028
Slezak D, Herndl GJ (2003) Effects of ultraviolet and visible radiation on the cellular concentrations of dimethylsulfoniopropionate (DMSP) in Emiliania huxleyi (strain L). Mar Ecol Prog Ser 246:61–71
Slezak D, Brugger A, Herndl GJ (2001) Impact of solar radiation on the biological removal of dimethylsulfoniopropionate and dimethylsulfide in marine surface waters. Aquat Microb Ecol 25:87–97
Slezak D, Kiene RP, Toole DA, Simó R, Kieber DJ (2007) Effects of solar radiation on the fate of dissolved DMSP and conversion to DMS in seawater. Aquat Sci 69:377–393
Smith D, Azam F (1992) A simple, economical method for measuring bacteria protein synthesis rates in seawater using 3H-leucine. Mar Microb Food Webs 6:107–114
Stefels J, Steinke M, Turner S, Malin G, Belviso S (2007) Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry 83:245–275
Straza TRA, Ducklow HW, Murray AE, Kirchman D (2010) Abundance and single-cell activity of bacterial groups in Antarctic coastal waters. Limnol Oceanogr 55:2526–2536
Sunda W, Kieber DJ, Kiene RP, Huntsman S (2002) An antioxidant function for DMSP and DMS in marine algae. Nature 418:317–320
Tripp HJ, Kitner JB, Schwalbach MS, Dacey JWH, Wilhelm LJ, Giovannoni SJ (2008) SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 452:741–744
Vila M, Simó R, Kiene RP, Pinhassi J, González JA, Moran MA, Pedrós-Alió C (2004) Use of microautoradiography combined with fluorescence in situ hybridization to determine dimethylsulfoniopropionate incorporation by marine bacterioplankton taxa. Appl Environ Microbiol 70:4648–4657
Vila-Costa M, Simó R, Harada H, Gasol JM, Slezak D, Kiene RP (2006) Dimethylsulfoniopropionate uptake by marine phytoplankton. Science 314:652–654
Vila-Costa M, Pinhassi J, Alonso C, Pernthaler J, Simó R (2007) An annual cycle of dimethylsulfoniopropionate-sulfur and leucine assimilating bacterioplankton in the coastal NW Mediterranean. Environ Microbiol 9:2451–2463
Vila-Costa M, Simó R, Alonso-Sáez L, Pedrós-Alió C (2008) Number and phylogenetic affiliation of bacteria assimilating dimethylsulfoniopropionate and leucine in the ice-covered coastal Arctic Ocean. J Marine Syst 74:957–963
Wickham S, Carstens M (1998) Effects of ultraviolet-B radiation on two arctic microbial food webs. Aquat Microb Ecol 16:163–171
Zubkov MV, Fuchs BM, Archer SD, Kiene RP, Amann R, Burkill PH (2002) Rapid turnover of dissolved DMS and DMSP by defined bacterioplankton communities in the stratified euphotic zone of the North Sea. Deep Sea Res Pt II 49:3017–3038
Acknowledgments
The authors thank the chief scientists of the ATOS I and II cruises, C. M. Duarte and J. Dachs, and all technicians and the crew aboard the BIO Hespérides for their assistance and cooperation. The authors also thank J. Felipe for his help with the processing of the flow cytometry and chlorophyll samples. The authors are especially indebted to R. P. Kiene (University of South Alabama) for kindly providing 35S-DMSP. Financial support for this study was provided by the projects MODIVUS (CTM2005-04795/MAR), SUMMER (CTM2008-03309/MAR) and ATOS (POL2006-00550/CTM) funded by the Spanish Ministry of Science and Innovation (MICINN). C.R.-G. the receipt of a FPI studentship from the MICINN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-González, C., Galí, M., Gasol, J.M. et al. Sunlight effects on the DMSP-sulfur and leucine assimilation activities of polar heterotrophic bacterioplankton. Biogeochemistry 110, 57–74 (2012). https://doi.org/10.1007/s10533-012-9699-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9699-y