Abstract
The effective conservation and management of threatened species requires comprehensive knowledge about resource utilisation. Here we integrated tissue stable isotope analysis and biotelemetry to identify the predominant dietary resources of two sympatric species of freshwater turtle, and locate where those items were acquired. We deployed an array of underwater acoustic telemetry receivers to autonomously, simultaneously, and continuously, monitor the movements of the threatened Elseya albagula and Elusor macrurus, over a 12-month period. Stable isotope (SI) values (δ13C and δ15N) were measured within the carapace of each species, and compared with SI values within potential food items. The integration of movement information and carapace SI data revealed that whilst these species had overlapping home ranges, there was less than 5% probability of inter-species dietary niche overlap. E. macrurus acquired food items consisting of bivalves, gastropods and aquatic insects within rocky riffles whilst E. albagula fed on filamentous algae and crustaceans foraged from the muddy and vegetated shallow margins of deep water pools. Our findings differ from stomach content analysis and mark-recapture studies, which reported these species to have similar habitat and resource requirements. We argue that the observed disparity is because our methods provided a weighted measure of an individual’s dietary preference and habitat utilisation over a broad time-scale, whilst stomach content analysis and mark-recapture studies offer only a single observation of an individual’s dietary preference. The research demonstrates the utility of integrating passive acoustic telemetry and carapace stable isotope analysis for identifying critical habitat for freshwater turtles.
Similar content being viewed by others
Introduction
The movement and occurrence of species generally reflects spatiotemporal patterns in the quantity and quality of resources (Hutchinson 1957; Tempel and Gutiérrez 2013). The effective conservation of threatened species requires comprehensive information about species distributions, and a large body of research has been focused around identifying the resources utilised by a species and predicting where these occur in the environment. To collect these types of data, tissue stable isotope analysis and biotelemetry devices have become popular research techniques (Dwyer et al. 2015; Campbell et al. 2015) The composition of stable isotopes in animal tissues reflects their diet, and since different tissues turnover at different rates, the isotopic composition of different tissues in the same animal can provide insight into diet over multiple time frames (Jardine et al. 2012; Hanson et al. 2015). Integrating dietary information with individual-based movement data, collected by animal-borne biotelemetry devices, can reveal the relative significance of habitats to an individual, population, or species (Cunjak et al. 2005; Papastamatiou et al. 2010; Matich et al. 2011; Caron-Beaudoin et al. 2013; Rosenblatt et al. 2015).
In marine turtle research, biotelemetry technologies are used extensively to collect high resolution location data upon individuals (Pajuelo et al. 2012). These data are then used to reveal the location of significant areas, critical habitats, and corridors (Stokes et al. 2014, 2015). Similarly, tissue stable isotope analysis is used routinely to assess dietary preferences and identify the location of marine turtle feeding grounds (Godley et al. 1998; Burkholder et al. 2011). The integration of diet and movement information has dramatically enhanced our understanding of marine turtle resource use, and has improved the conservation and management for many populations (Brooks et al. 2009; Bailleul et al. 2010; Ceriani et al. 2012; MacDonald et al. 2012, 2013; Pajuelo et al. 2012; Scales et al. 2011; Thums et al. 2013).
In contrast, automated biotelemetry locating technologies and tissue stable isotope analysis have been seldom used to identify critical habitat and resource use in freshwater turtles (e.g. Seminoff et al. 2007; Bulte and Blouin-Demers 2008; Aresco 2010; Lara et al. 2012; Pearson et al. 2013; Micheli-Campbell et al. 2013; Christensen and Chow-Fraser 2014; Markle and Chow-Fraser 2014). In freshwater turtle research, movement and habitat preference data are typically collected by direct observation, mark-recapture, or active tracking using VHF radio (Georges 1982; Eckler et al. 1990; Buhlmann and Vaughan 1991; Slavenko et al. 2016). These methods provide irregular and infrequent location fixing, and produce data that are not effective for identifying high-utilisation areas at the fine spatial scale (Kie et al. 2010). To assess diet in freshwater turtles’ stomach flushing and then analysis of the content remains the most common method (Spencer et al. 2014). This is despite a growing body of literature that asserts that a single measure of the stomach contents does not provide reliable information for understanding the trophic niche requirements of an individual or population (Marques et al. 2011).
It is not immediately apparent why research methods used routinely for assessing diet and habitat preference in marine turtles have not been similarly applied to freshwater turtles. It may simply be that automated biotelemetry devices and tissue stable isotope analysis are more expensive to implement than mark/recapture, VHF telemetry, and stomach flushing; and considered beyond the financial limits of freshwater turtle conservation and management programs. The costs of autonomous biotelemetry devices and tissue stable isotope analysis has however, reduced substantially in recent years, and because freshwater turtle populations are in severe decline (Hoffmann et al. 2010), we considered it important to assess if the application of these research techniques enhanced our understanding of critical habitat in freshwater turtles.
Here we assessed the resources used by two species of sympatric and threatened species of freshwater turtle, Elusor macrurus and Elseya albagula. We used animal-borne acoustic transmitters with depth-sensors and fixed underwater receivers to ascertain the location of high-use foraging areas at the fine spatial scale. These data were integrated with a broad temporal-scale assessment of dietary preference, by comparing stable isotope values within the turtle carapace with potential food items. The findings were compared with current knowledge about resource for these species, gathered through the traditional sampling techniques of stomach flushing, mark-recapture, and active location fixing using VHF-radio telemetry.
Materials and methods
Study area
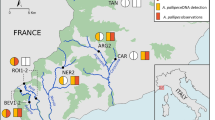
The study was conducted in the mid-catchment stretch of the Mary River, Queensland, Australia (Fig. 1). This stretch was selected because both E. macrurus and E. albagula have been captured here (Limpus 2008). The river stretch is composed of a sequence of riffle (depth = 0.5–1 m; flow < 1.5 ms−1) and pool sections (depth 3–6 m; flow < 0.2 ms−1), an arrangement that is characteristic of the Mary River (Pusey et al. 2004; Kennard et al. 2006).
Turtle capture
Turtles were captured using double-winged set-nets. The nets were deployed at six locations throughout a 4.7 km river stretch, within water of 1–1.5 m depth. The net design resulted in the funnelling of turtles moving along the river into a 4 m length × 1 m diameter cod-end. Once in the cod-end, the turtles could move freely and surface to breathe. A total of 16 adult female E. macrurus (body mass 3.4 ± 0.3 kg, carapace length 313 ± 8 mm, mean ± SE) and 15 adult female E. albagula (body mass 4.19 ± 0.15 kg, carapace length 327 ± 5 mm, mean ± SE) were captured using this method.
Biotelemetry
Five female adults of each species had acoustic transmitters (V13TP; VEMCO, Halifax, NS, Canada) attached to their carapaces. The transmitters were 36 L × 13 D mm, weighed 11 g in air (<1% of the turtle’s body mass), and had an in-build depth sensor (accurate to 0.1 m). The recorded depth data and a unique ID code for each turtle was emitted as a sonic pulse every 60 s at an output of 147 dB. The transmission frequency and power output was optimised to provide battery longevity of ~12 months. The acoustic transmitters were attached to the posterior marginal scutes of the carapace using a purpose built cap, plastic saddle, and PVC nuts and bolts (1.5 mm). The transmitter was attached to the carapace via two holes (2.5 mm diameter) drilled vertically though the marginal scutes. The bolts were inserted through the holes on the underside of the carapace and fixed with a washer and nut. The nuts were secured with Loctite 243 (Henkel, Victoria, Australia), and the ends of the bolts were covered in a two-part epoxy putty (KneadIt, Selleys, Padstow, Australia) to prevent abrasion with the turtles’ skin.
Twelve static underwater hydrophones (VR2-W; VEMCO) were deployed along a 5.5 km stretch of river to detect the acoustic pulses emitted from the transmitters. The receiver array encompassed the trapping area, plus four riffles and three pooled stretches of river (Fig. 1). The hydrophones were spaced every 500 ± 50 m, receiver spacing was dependant on the availability of suitable deployment locations. Each hydrophone was secured to a concrete anchor (15 kg) and moored to the riverbank using 6 mm multi-strand stainless steel cable. The detection range of each hydrophone was between 150 and 350 m, depending on river topography. The detection distance of each hydrophone was determined by towing an activated transmitter behind a boat away from each hydrophone, and comparing the received and missing detections with the geographic location of the boat as determined by GPS (680, Garmin USA). The hydrophones were deployed in October 2012 and retrieved in October 2013.
Stable isotope analysis
Stable isotope analysis was performed on the carapace of 11 E. macrurus and 10 E. albagula. The sample area was cleaned of biofilm and algae, and the core extracted using a standard drill (Ryobi, HRD, Japan) fitted with a 3 mm bonded diamond drill bit for porcelain (DiamondDrillandTool.com). The core was immediately placed on ice and then stored in a laboratory freezer at −20 °C within 24 h.
Tissue samples were also collected from a wide range of potential turtle food sources within the study area using standard methods detailed in Jardine et al. (2012). These included terrestrial vegetation, filamentous algae, epilithic biofilm (representing a mixture of periphyton and detritus), seston (likely including phytoplankton, organic detritus, and inorganic material), molluscs (bivalves and gastropods), aquatic insects (simuliids, corixids, ephemeropterans, trichopterans) and crustaceans (atyids and macrobranchium). Seston samples (n = 4, replicate samples) were filtered using a pre-combusted filter, and a hand pump system. Each sample was filtered from at least 500 ml of water. Filamentous algae samples (n = 7) were collected by hand. Epilithic biofilm samples (n = 6) were scraped from rinsed rocks with a scalpel and stored in 5 ml sterile plastic tubes. The bivalves (n = 6) and gastropods (n = 6) were collected by sieving the substrate. Aquatic insects (n = 18) were collected using a dip net (mesh size 250 µm). Crustaceans (n = 12) were collected by seine net (mesh size 11 mm), and dip net. Samples were immediately placed in individually labelled zip-lock bags, or in 5 ml tubes, and then stored on ice. Samples were subsequently frozen for transportation and storage at −20 °C in a laboratory freezer.
Following standard stable isotope sample preparation and analysis protocols (see Fry 2006; Jardine et al. 2012), all turtle food source and turtle carapace samples were defrosted, rinsed with distilled water, and then dried in an oven (60 °C for at least 24 h). They were then ground to a fine powder in an electric ball-mill grinder (Retsch MM200, Haan, Germany). The resultant mixture was weighed, transferred into small tin capsules and analysed for C and N isotopes. Isotope analyses were performed via combustion and mass spectrometry using a Sercon Europa EA-GSL inlet with a Sercon Hydra 20-22 isotope ratio mass spectrometer, at the Australian Rivers Institute Stable Isotope Laboratory. Stable isotope values of samples were calculated as parts per thousand (‰) relative to international standards (Pee Dee Belemnite carbonate and atmospheric nitrogen). Lipid correction was not performed on δ13C values, as most samples had C/N ratios <4, which are indicatives of low lipid content (Jardine et al. 2012). Machine measurements were precise, within ± 0.1‰ SD for δ13C, and ± 0.1‰ SD for δ15N, respectively.
Data analysis
Acoustic detection data were downloaded and converted into durations of time when tagged turtles were within (residence event) and between the detection fields of each hydrophone (movement event). This was performed in the V-Track package (Campbell et al. 2012) in R (R Core Team 2016). These data were used to determine movement and home-range parameters, following methods in Dwyer et al. (2015). The centroid point of each turtle home-range was determined using the Calculate Geometry tool in ESRI ArcGIS 10.2.2.
Generalised linear mixed effects models were used to test for species differences in vertical occurrence and activity. The depth recorded by the transmitter sensor was assigned the dependent variable, time and body mass as co-variables, species as factor, and individual ID as the random effect in the model. Depth data were log-transformed to ensure linearity. Significance of dependent variables were assessed by dropping parameters from the full model and applying a deviance test, where P < 0.05 indicated that the variable was a significant predictor of horizontal activity (Zuur et al. 2009).
The stable isotope values (δ13C and δ15N) for each individual and potential turtle food sources were plotted in two-dimensions. Minimum convex polygons were drawn around isotopic values for each species, and used to visually represent dietary overlap between species and infer potential food sources. Significant differences in isotopic values between species were examined using One-Way ANOVA. To quantify the isotopic niche for each species, and the degree of inter-species overlap, a probabilistic analysis was performed with two-dimensional stable isotope data (δ13C andδ15N) using the nicheROVER package in R (details in Swanson et al. 2015). This multivariate approach incorporated statistical uncertainty through a Bayesian framework, and did not assume the uniform distribution of individuals within the niche region when calculating the degree of overlap between species. Therefore, the technique is applicable to the small (>10) and unequal sample sizes used in this study. We defined the degree of inter-species niche overlap as the probability that the isotopic niche from an individual E. macrurus would be located within the isotopic niche of E. albagula, and vice versa.
Results
Movement and space-use
The location of each of the tagged turtles was recorded continuously over 12 months. The average number of days tracked per turtle was 342 ± 0.21 days (mean ± SE). A total of 443,942 acoustic detections (263,563 E. macrurus; 180,379 E. albagula) were collected across the 11 hydrophones within the array (Table 1), with an average of 44,394 ± 9045 acoustic detections per turtle. E. macrurus travelled a five-fold greater daily distance along the river when compared to E. albagula, with 80% of E. macrurus moving >1 km river distance per day. This increased daily movement resulted in E. macrurus having a significantly larger linear home-range (Table 1; Fig. 2; f = 15.8; df = 1,6; P < 0.01) compared to E. albagula. The activity centre of the home-range for E. macrurus was within the river riffles, whilst that of E. albagula was outside the riffles and of a distance placing the home-range centroid in slow moving pooled water sections of the river (Table 1).
Individuals from both species spent the majority of their underwater activity at depths ≥ 2 m. All individuals did occasionally dive to depths greater than 6 m (Fig. 3). Over the 24-h period, there were significant inter-species differences in the maximum depth profile attained whilst diving (Table 2). The highest proportion of dives undertaken by E. albagula were between depths of 1 and 2 m. E. macrurus showed a bimodal distribution, undertaking dives to depths of less than 1 m but also dived frequently to depths between 4 and 6 m. Combining the vertical depth data with the horizontal movement information showed that E. macrurus spent the largest proportion of shallow dives within riffles. In contrast, the shallow dives undertaken by E. albagula were within the ponded slow-moving sections of river (Table 1; f = 166; df = 1,6; P < 0.01). Surveys of the river substratum around the home-range activity centres, showed that E. macrurus dived in areas with a substratum composed of large boulders (>0.5 m diameter), whilst at the centroid of the home-range for E. albagula the substratum was composed of a muddy, sandy, leaf litter, or vegetated substratum.
Trophic ecology
Stable isotope analysis of the carapace revealed that E. macurus had significantly lower δ13C and higher δ15N isotopic values than E. albagula. This indicated inter-species differences in dietary carbon sources and trophic position between the two species (Table 3). E. macrurus had a greater intra-species variation in δ13C compared to E. albagula, but intra-species variation of δ15N was similar between species. Visual comparison of turtle carapace stable isotopic values with potential food sources (Fig. 4) illustrated that E. macurus had a greater reliance on seston and biofilm carbon sources and likely foraged on bivalves, gastropods, aquatic insects and crustaceans (for some individuals). In contrast, the comparatively higher carbon isotope values for E. albagula were more closely matched to those of filamentous algae, crustaceans and gastropods. Assessment of the isotopic niche across the two dimensions of δ13C and higher δ15N showed that the probability that E. macrurus fed within the dietary niche of the E. albagula population was 3%, and the probability that E. albagula fed from within the dietary niche of the E. macrurus population was 5% (Fig. 5).
Stable isotope ratios of δ13C and δ15N of carapace scutes from E. albagula (n = 10; open circles) and E. macrurus (n = 11; closed circles). Dashed lines show minimum convex polygons encompassing the range of variation in δ13C and δ15N for each species. Mean stable isotope ratios (δ13C and δ15N) of potential turtle food sources (±1SE) are shown
Discussion
The sympatric freshwater turtle species E. macrurus and E. albagula are reported to have similar dietary and habitat requirements (Cann 1998; Rogers 2000; Tucker 2000; Flakus 2002; Thomson et al. 2006; Limpus 2008; Tucker et al. 2012; Department of the Environment 2016a, b). Contrary to these observations, this study found these species fed predominantly on different resources, located in discrete areas of the river, and characterised by distinct environmental conditions. We argue that the inter-study discrepancies are because the methods used in this study provide a weighted measure of dietary preference and habitat utilisation over a broad timescale, whilst stomach content analysis and mark-recapture provide only a single observation of an individual’s diet and occurrence. We discuss the potential impact of these findings for freshwater turtle conservation and management.
The application of passive acoustic telemetry to assess the habitat utilisation of freshwater turtles is not common in the literature. The technology was designed for monitoring fish, and application of this technology in fisheries research and management remains its primary use. Here we demonstrated that this equipment is easily adapted to effectively monitor habitat utilisation and movement in freshwater turtles. The transmitters can be attached externally to the carapace without impediment to the turtle or risk of entanglement. The relatively small home-ranges exhibited by freshwater turtles, and their low rate of daily movement, allows for individual turtles to be detected continuously, and simultaneously, over long periods of time. In this study, the collective detection data across the receiver array were of sufficient frequency, duration, and resolution, to reveal finite inter-species differences in high-usage areas of the river. We argue that this finding may not have been observed with less frequent location sampling because these species do have overlapping home-ranges. The acoustic transmitters also recorded and transmitted the water depth of the tagged turtles, allowing vertical movements to be recorded simultaneously with the horizontal movements. Using these data, it was possible to observe 1/areas where the turtles foraged, 2/areas where they rested, and 3/areas which the turtles merely moved through (Gordos et al. 2007; Campbell et al. 2010).
We discovered that δ13C levels within the carapace differed significantly between E. macrurus and E. albagula. In river systems, δ13C generally varies in a predictable manner with water velocity, with more depleted δ13C being predominant within slow moving stretches of water and enriched δ13C within faster flowing stretches (Finlay et al. 2002). The fact that E. albagula had more depleted δ13C within the carapace compared to E. macrurus suggests E. albagula fed within river stretches with slower moving water than did E. macrurus. This also supports the acoustic telemetry data, which showed there was inter-species segregation in foraging locations. Comparison of the carapace stable isotope values with potential food items revealed E. macrurus fed predominantly on bivalves, aquatic insects, and gastropods, and E. albagula fed primarily on crustaceans and filamentous algae. These observations are in contrast to stomach content analyses, which reported both species to feed primarily on aquatic plants (79%), with terrestrial plants and aquatic invertebrates only comprising a minor component of the diet (Rogers 2000; Tucker 2000; Flakus 2002; Tucker et al. 2012; Thomson et al. 2006). The contrasting results likely relates to the temporal scale over which the observations were made. Stomach content analysis is a single snap-shot of recently ingested items, whilst the stable isotopic values within the turtle carapace have been laid down over many years (Van der Zanden et al. 2010).
Dietary studies of other sympatric freshwater turtle species have also relied upon the stomach content analysis method (Vogt and Guzman 1988; Allanson and Georges 1999; Rogers 2000; Tucker 2000; Flakus 2002; Thomson et al. 2006; Tucker et al. 2012; Spencer et al. 2014). Interestingly, these studies also reported the sympatric species of study to feed on similar resources. We argue that these observations may be artefacts of the methodology, which may be influenced by seasonal shifts in resources, rates of digestion, and nutrient assimilation (Marques et al. 2011). Moreover, the observation that sympatric species of freshwater turtle feed on the same resources is at odds with the competitive exclusion principal (Hardin 1960). Therefore, we suggest that researchers assessing dietary preferences in freshwater turtles integrate stomach content analysis with assessment of stable isotopes values within the carapace, because this will provide a more complete understanding of dietary preferences over the turtle’s long life time.
Management implications
The methods used in this study were a higher cost to implement than mark-recapture, VHF telemetry and stomach content analysis techniques. To ensure that limited conservation dollars are used effectively it is important to assess if this new information would aid in the formulation of management decisions and improved conservation for the species. Below we have assessed the novel findings from this study with what is currently known for these species.
E. macrurus is currently listed as ‘endangered’ both nationally (Department of the Environment 2016a) and internationally (IUCN 2015). This study does not alter the listing status of E. macrurus because the methods did not provide evidence of changes in geographic distribution or population size. Instead the study showed that E. macrurus had a high dietary dependence on riffle-dwelling food items and used deep water pools for resting. Therefore, management actions for this species should focus on conserving riffle-pool sequences, and ensuring water conditions are suitable for the riffle dwelling fauna this study identified E. macrurus to predominately feed upon.
E. albagula is currently listed nationally as ‘critically endangered’, based upon an observed lack of juvenile recruitment into the population (Department of the Environment 2016b). The species has not yet been evaluated for listing by the IUCN. The findings from this study do not support or oppose the listing but do contrast with some of the current reports about E. albagula biology. Impoundments have been proposed as a major contributor to the species decline because they obstruct migration to nesting sites (Limpus 2008; Department of the Environment 2016b). Our data does not support a nesting migration of E. albagula, and the adult females tagged in this study either did not nest or nested locally during the 12-month study. Further telemetry work on E.albagula on other river stretches is required to reassess the migration theory. It is suggested that E. albagula are dependent on fast flowing well-oxygenated water (Limpus 2008; Department of the Environment 2016b). In contrast, the E. albagula tagged in this study foraged in the shallow margins of slow moving pooled water, they rested in deep slow moving water, and rarely moved through or occupied fast-flowing water. Therefore, they did not seem dependant on habitats consisting of fast-flowing well-oxygenated water. The absence of E. albagula from standing water bodies (Hamann et al. 2007) requires further investigation because this study does not suggest it to be dietary related.
The evidence from this study highlights the utility of continuous location monitoring via acoustic telemetry and carapace stable isotope analysis for determining resource use in long-lived animals, such as freshwater turtles. Integrating these methods provided a weighted measure of dietary resource acquisition. This was measured over a suitably broad temporal scale to identify threatening processes, whilst at an appropriately fine spatial scale to direct management actions.
References
Allanson M, Georges A (1999) Diet of Elseya purvisi and Elseya georgesi (Testudinata: chelidae), a sibling species pair of freshwater turtles, from eastern Australia. Chelonian Conserv Biol 3:473–477
Aresco MJ (2010) Competitive interactions of two species of freshwater turtles, a generalist omnivore and herbivore, under low resource conditions. Herpetologica 66:259–268
Bailleul F, Authier M, Ducatez S, Roquet F, Charrassin JB, Cherel Y, Guinet C (2010) Looking at the unseen: combining animal bio-logging and stable isotopes to reveal a shift in the ecological niche of a deep diving predator. Ecography 33:709–719
Brooks LB, Harvey JT, Nichols WJ (2009) Tidal movements of East Pacific green turtle Chelonia mydas at a foraging area in Baja California Sur, Mexico. Mar Ecol Prog Ser 386:263–274
Buhlmann KA, Vaughan MR (1991) Ecology of the turtle Pseudemys concinna in the New River, West Virginia. J Herpetol 25:72–78
Bulte G, Blouin-Demers G (2008) Northern map turtles (Graptemys geographica) derive energy from the pelagic pathway through predation on zebra mussels (Dreissena polymorpha). Freshwater Biol 53:497–508
Burkholder DA, Heithaus MR, Thomson JA, Fourqurean JW (2011) Diversity in trophic interactions of green sea turtles Chelonia mydas on a relatively pristine coastal foraging ground. Mar Ecol Prog Ser 439:277–293
Campbell HA, Sullivan S, Read MA, Gordos MA, Franklin CE (2010) Ecological and physiological determinants of dive duration in the freshwater crocodile. Funct Ecol 24:103–111
Campbell HA, Watts ME, Dwyer RG, Franklin CE (2012) V-Track: software for analysing and visualising animal movement from acoustic telemetry detections. Mar Freshw Res 63:815–820
Campbell HA, Beyer HL, Dennis TE, Dwyer RG, Forester JD, Fukuda Y et al (2015) Finding our way: on the sharing and reuse of animal telemetry data in Australasia. Sci Total Environ 534:79–84
Cann J (1998) Australian freshwater turtles. John Cann & Beaumont Publishing Pte Ltd., La Perouse
Caron-Beaudoin E, Gentes ML, Patenaude-Monette M, Helie JF, Giroux JF, Verreault J (2013) Combined usage of stable isotopes and GPS-based telemetry to understand the feeding ecology of an omnivorous bird, the Ring-billed gull (Larus delawarensis). Can J Zool 91:689–697
Ceriani SA, Roth JD, Evans DR, Weishampel JF, Ehrhart LM (2012) Inferring foraging areas of nesting loggerhead turtles using satellite telemetry and stable isotopes. PLoS ONE 7:12
Christensen RJ, Chow-Fraser P (2014) Use of GPS loggers to enhance radio-tracking studies of semi-aquatic freshwater turtles. Herpetol Conserv Biol 9:18–28
Cunjak RA, Roussel JM, Gray MA, Dietrich JP, Cartwright DF, Munkittrick KR, Jardine TD (2005) Using stable isotope analysis with telemetry or mark-recapture data to identify fish movement and foraging. Oecologia 144:636–646
Department of the Environment (2016a) Elusor macrurus in species profile and threats database, Department of the Environment, Canberra. Available from: http://www.environment.gov.au/sprat. Accessed 25 Jan 2016
Department of the Environment (2016b) Elseya albagula in species profile and threats database, Department of the Environment, Canberra. http://www.environment.gov.au/sprat. Accessed 25 Jan 2016
Dwyer RG, Campbell HA, Irwin TR, Franklin CE (2015) Does the telemetry technology matter? Comparing estimates of aquatic animal space-use generated from GPS-based and passive acoustic tracking. Mar Freshw Res 66:654–664
Eckler JT, Breisch AR, Behler JL (1990) Radio telemetry techniques applied to the bog turtle (Clemmys muhlenbergii, Schoepff 1801). New York State Museum, Albany
Finlay JC, Khandwala S, Power ME (2002) Spatial scales of carbon flow in a river food web. Ecology 83:1845–1859
Flakus SP (2002) Ecology of the Mary River turtle, Elusor macrurus. Masters Thesis, The University of Queensland
Fry B (2006) Stable Isotope Ecology. Springer, New York
Georges A (1982) Diet of the Australian freshwater turtle Emydura krefftii (Chelonia: Chelidae), in an unproductive lentic environment. Copeia 1982:331–336
Godley BJ, Thompson DR, Waldron S, Furness RW (1998) The trophic status of marine turtles as determined by stable isotope analysis. Mar Ecol Prog Ser 166:277–284
Gordos MA, Hamann M, Schauble CS, Limpus CJ, Franklin CE (2007) Diving behaviour of Elseya albagula from a naturally flowing and hydrologically altered habitat. J Zool 272:458–469
Hamann M, Schauble CS, Limpus DJ, Emerick SP, Limpus CJ (2007) Management plan for the conservation of Elseya sp. (Burnett River) in the Burnett River Catchment. Report to the Queenslnd Environmental Protection Agency. ISBN 978-0-9803613-0-8
Hanson JO, Salisbury SW, Campbell HA, Dwyer RG, Jardine TD, Franklin CE (2015) Feeding across the food web: the interaction between diet, movement, and body size. Austral Ecol 40(3):275–286
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297
Hoffmann M, Hilton-Taylor C, Angulo A, Bohm M, Brooks TM, Butchart SHM, Carpenter KE et al (2010) The impact of conservation on the status of the world’s vertebrates. Science 330:1503–1509
Hutchinson GE (1957) Cold spring harbor symposium on quantitative biology. Concluding Remarks 22:415–427
IUCN 2015. The IUCN Red List of threatened species. Version 2015-4. http://www.iucnredlist.org
Jardine TD, Pettit NE, Warfe DM, Pusey BJ, Ward DP, Douglas MM, Davies PM, Bunn SE (2012) Consumer-resource coupling in wet-dry tropical rivers. J Anim Ecol 81:310–322
Kennard MJ, Pusey BJ, Harch BD, Dore E, Arthington AH (2006) Estimating local stream fish assemblage attributes: sampling effort and efficiency at two spatial scales. Mar Freshw Res 57:635–653
Kie JG, Matthiopoulos J, Fieberg J, Powell RA, Cagnacci F, Mitchell MS, Gaillard JM, Moorcroft PR (2010) The home-range concept: are traditional estimators still relevant with modern telemetry technology? Philos Trans Soc B 365:2221–2231
Lara NRF, Marques TS, Montelo KM, de Ataides AG, Verdade LM, Malvasio A, de Camargo PB (2012) A trophic study of the sympatric Amazonian freshwater turtles Podocnemis unifilis and Podocnemis expansa (Testudines, Podocnemidae) using carbon and nitrogen stable isotope analyses. Can J Zool 90:1394–1401
Limpus CJ (2008) Freshwater turtles in the Mary River: review of biological data for turtles in the Mary River, with emphasis on Elusor macrurus and Elseya albagula). Queensland Government, Brisbane. https://www.ehp.qld.gov.au/wildlife/pdf/mary-river-turtles.pdf
MacDonald BD, Lewison RL, Madrak SV, Seminoff JA, Eguchi T (2012) Home ranges of East Pacific green turtles Chelonia mydas in a highly urbanized temperate foraging ground. Mar Ecol Prog Ser 461:211–221
MacDonald BD, Madrak SV, Lewison RL, Seminoff JA, Eguchi T (2013) Fine scale diel movement of the east Pacific green turtle, Chelonia mydas, in a highly urbanized foraging environment. J Exp Mar Biol Ecol 443:56–64
Markle CE, Chow-Fraser P (2014) Habitat selection by the Blanding’s turtle (Emydoidea blandingii) on a protected island in Georgian Bay, Lake Huron. Chelonian Conserv Biol 13:216–226
Marques TS, Tassoni M, Ferronato BO, Guardia I, Verdade LM, de Camargo PB (2011) Isotopic signatures (delta C-13 and delta N-15) of muscle, carapace and claw in Phrynops geoffroanus (Testudines: Chelidae). Zoologia 28:407–410
Matich P, Heithaus MR, Layman CA (2011) Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J Anim Ecol 80:294–305
Micheli-Campbell MA, Campbell HA, Connell MJ, Dwyer RG, Franklin CE (2013) Integrating telemetry with a predictive model to assess habitat preferences and juvenile survival in an endangered freshwater turtle. Fresh Biol 58:2253–2263
Pajuelo M, Bjorndal KA, Reich KJ, Arendt MD, Bolten AB (2012) Distribution of foraging habitats of male loggerhead turtles (Caretta caretta) as revealed by stable isotopes and satellite telemetry. Mar Biol 159:1255–1267
Papastamatiou YP, Friedlander AM, Caselle JE, Lowe CG (2010) Long-term movement patterns and trophic ecology of blacktip reef sharks (Carcharhinus melanopterus) at Palmyra Atoll. J Exp Mar Biol Ecol 386:94–102
Pearson SH, Avery HW, Kilham SS, Velinsky DJ, Spotila JR (2013) Stable isotopes of C and N reveal habitat dependent dietary overlap between native and introduced turtles Pseudemys rubriventris and Trachemys scripta. PLoS ONE 8:e62891
Pusey BJ, Kennard MJ, Arthington AH (2004) Freshwater fishes of North-Eastern Australia. CSIRO Publishing, Collingwood
R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Rogers VM (2000) Dietary ecology including dietary resource partitioning of four species of chelid turtle in a tributary of the Fitzroy River, central Queensland. Honours Thesis, Central Queensland University
Rosenblatt A, Nifong J, Heithaus M, Mazzotti F, Cherkiss M, Jeffery B, Elsey R et al (2015) Factors affecting individual foraging specialization and temporal diet stability across the range of a large “generalist” apex predator. Oecologia 178:5–16
Scales KL, Lewis JA, Lewis JP, Castellanos D, Godley BJ, Graham RT (2011) Insights into habitat utilisation of the hawksbill turtle, Eretmochelys imbricata (Linnaeus, 1766), using acoustic telemetry. J Exp Mar Biol Ecol 407:122–129
Seminoff JA, Bjorndal KA (2007) Bolten AB (2007) Stable carbon and nitrogen isotope discrimination and turnover in pond sliders Trachemys scripta: insights for trophic study of freshwater turtles. Copeia 3:534–542
Slavenko A, Itescu Y, Ihlow F, Meiri S (2016) Home is where the shell is: predicting turtle home range sizes. J Anim Ecol 85:106–114
Spencer RJ, Georges A, Lim D, Welsh M, Reid AM (2014) The risk of inter-specific competition in Australian short-necked turtles. Ecol Res 29:767–777
Stokes KL, Fuller WJ, Glen F, Godley BJ, Hodgson DJ, Rhodes KA, Snape RTE, Broderick AC (2014) Detecting green shoots of recovery: the importance of long-term individual-based monitoring of marine turtles. Anim Conserv 17:593–602
Stokes KL, Broderick AC, Canbolat AF, Candan O, Fuller WJ, Glen F, Levy Y et al (2015) Migratory corridors and foraging hotspots: critical habitats identified for Mediterranean green turtles. Divers Distrib 21:665–674
Swanson HK, Lysy M, Power M, Stasko AD, Johnson JD, Reist JD (2015) A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 96:318–324
Tempel DJ, Gutiérrez RJ (2013) Relation between occupancy and abundance for a territorial species, the California spotted owl. Conserv Biol 27:1087–1095
Thomson S, Georges A, Lijmpus CJ (2006) A new species of freshwater turtle in the genus Elseya (Testudines: Chelidae) from central coastal Queensland, Australia. Chelonian Conserv Biol 5:74–86
Thums M, Whiting SD, Reisser JW, Pendoley KL, Pattiaratchi CB, Harcourt RG, McMahon CR, Meekan MG (2013) Tracking sea turtle hatchlings - A pilot study using acoustic telemetry. J Exp Mar Biol Ecol 440:156–163
Tucker AD (2000) Cumulative effects of dams and weirs on freshwater turtles: Fitzroy, Kolan, Burnett and Mary catchments. Queensland Department of Natural Resources, Brisbane
Tucker AD, Guarino F, Priest TE (2012) Where lakes were once rivers: contrasts of freshwater turtle diets in dams and rivers of Southeastern Queensland. Chelonian Conserv Biol 11:12–23
Vander Zanden HB, Bjorndal KA, Reich KJ, Bolten AB (2010) Individual specialists in a generalist population: results from a long-term stable isotope series. Biol Lett 6:711–714
Vogt RC, Guzman SG (1988) Food partitioning in a neotropical freshwater turtle community. Copeia 1988:37–47
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This research was funded by an International Postgraduate Research Scholarship (IPRS) awarded to M.A.M.C. by the Australian Federal Government and The University of Queensland, an Australian Research Council Linkage Grant to CEF (LP0992220) with Tiaro & District Landcare Group as industry partners, a Queensland Government Everyone’s Environment Grant, and support from SEQwater. All procedures were conducted in accordance with The University of Queensland Animal Ethics Committee (SBS/301/12/URG) and a Queensland Department of Environment and Heritage Scientific Purposes Permit (WISP02255909).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dirk Sven Schmeller.
Rights and permissions
About this article
Cite this article
Micheli-Campbell, M.A., Connell, M.J., Dwyer, R.G. et al. Identifying critical habitat for freshwater turtles: integrating long-term monitoring tools to enhance conservation and management. Biodivers Conserv 26, 1675–1688 (2017). https://doi.org/10.1007/s10531-017-1325-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-017-1325-9