Abstract
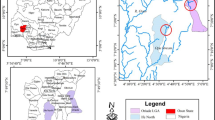
The socio-economic impacts of the free-floating aquatic plant water hyacinth, Eichhornia crassipes (Pontederiaceae), on aquatic systems are well documented, yet the impacts on aquatic biodiversity, particularly invertebrate biodiversity, are less well understood. This study aimed to determine whether the presence of water hyacinth altered the diversity and assemblage structure of benthic macroinvertebrates in a conservation area. The benthic macroinvertebrate assemblage was sampled over 1 year at five sites under water hyacinth mats and at five sites without water hyacinth at Lake Nsezi—Nseleni River in the vicinity of Richards Bay, KwaZulu-Natal, South Africa. Artificial substrates were placed beneath water hyacinth mats or in the open water to allow for colonization by freshwater macroinvertebrates, and left for a period of 6 weeks, repeated on seven occasions. Twenty nine families comprising 18,797 individuals were collected, 817 (13 families) individuals were from under water hyacinth mat sites compared to 17,980 (27 families) individuals from open water sites. Ninety-eight percent of individuals collected were, however, the invasive snail, Tarebia granifera. Open water samples were separated from samples beneath the water hyacinth mat by non-metric Multidimensional Scaling, indicating reduced biodiversity associated with the presence of water hyacinth. Exclusion of the dominant Thiaridae from the analysis did not alter the groupings. Family richness(s) and abundance (N) were significantly higher in open water communities(S: H3 = 21.09; P = 0.0001; N: H3 = 22.58; P = 0.00001), while evenness (J’) was higher under water hyacinth mats (H3 = 20.13; P = 0.0002). The presence of water hyacinth had a significantly negative impact on aquatic macroinvertebrate biodiversity in a conservation area, and therefore the control of this invasive aquatic plant must play a major role in catchment management.



Similar content being viewed by others
References
Appleton CC, Nadasan DS (2002) First report of Tarebia granifera (Gastropoda: Thiaridae) from Africa. J Mollus Stud 68:399–402
Appleton CC, Forbes AT, Demetriades NT (2009) The occurrence, bionomics and potential impacts of the invasive freshwater snail Tarebia granifera (Gastropoda: Thiaridae) in South Africa. Zool Meded 83:525–536
Brendonck L, Maes J, Rommens W, Dekeza N, Nhiwatiwa T, Barson M, Callebaut V, Phiri C, Moreau K, Gratwicke B, Stevens M, Alyn N, Holsters E, Ollevier F, Marshall B (2003) The impact of water hyacinth (Eichhornia crassipes) in a eutrophic subtropical impoundment (Lake Chivero, Zimbabwe). II. Species diversity. Arch Hydrobiol 158:389–405
Center TD, Hill MP, Cordo H, Julien MH (2002) Water hyacinth. In: van Driesche RG, Lyon S, Blossey B, Hoddle MS, Reardon R (eds) Biological control of invasive plants in the Eastern US. USDA Forest Service, Morgantown, pp 41–64
Chazdon RL, Colwell RK, Denslow JS, Guariguata MR (1998) Statistical methods for estimating species richness of woody regeneration of primary and secondary rain forests of northeastern Costa Rica. In: Dallmeier F, Comiskey JA (eds) Forest biodiversity research, monitoring and modeling: conceptual background and old world case studies. Parthenon Publishing, Paris, pp 285–309
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Coetzee JA, Hill MP, Julien MH, Center TD, Cordo HA (2009) 11. Eichhornia crassipes. In: Muniappan R, Reddy GVP, Raman A, Gandhi VP (eds) Biological control of tropical weeds using arthropods. Cambridge University Press, Cambridge, pp 181–208
Coetzee JA, Hill MP, Byrne MJ, Bownes AB (2011) A review of the biological control programmes on Eichhornia crassipes (C. Mart.) Solms (Pontederiacaeae), Salvinia molesta (Salviniaceae), Pistia stratiotes L. Myriophyllum aquaticum (Vell.) Verdc. and Azolla filiculoides(Azollaceae) in South Africa since 1999. Afr Entomol 19(2):451–468
Colwell RK (2005) EstimateS: statistical estimation of species richness and shared species from samples. Version 8.0. User’s Guide and application. http://purl.oclc.org/estimates. Accessed Jun 15 2013
Cook CDK (2004) Aquatic and wetland plants of Southern Africa. Backhuys Publishers, Leiden
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems. Bioscience 49:119–127
Davies BR, Day J (1998) Vanishing waters. University of Cape Town Press, Cape Town
Day JA, De Moor IJ (2002) Guides to the Freshwater Invertebrates of Southern Africa. Volume 6: Arachnida and Mollusca. WRC Report No. TT 182/02, pp. 141
Day JA, Harrison AD, De Moor IJ (2003) Guides to the Freshwater Invertebrates of Southern Africa. Volume 9: Diptera. WRC Report No. TT 201/02, pp. 200
De Moor IJ, Day JA, De Moor FC (2003a) Guides to the Freshwater Invertebrates of Southern Africa. Volume 7: Insecta I. WRC Report No. TT 207/03, pp. 286
De Moor IJ, Day JA, De Moor FC (2003b) Guides to the Freshwater Invertebrates of Southern Africa. Volume 8: Insecta II. WRC Report No. TT 214/03, pp. 209
Dickens C, Graham M (2002) The South African Scoring System (SASS) Version 5. Rapid bioassessment method for Rivers. Afr J Aquat Sci 27:1–10
Edwards D, Musil CJ (1975) Eichhornia crassipes in South Africa—a general review. J Limnol Soc S Afr 1:23–27
Gerber A, Gabriel MJM (2002) Aquatic invertebrates of South African Rivers: field guide. Department of Water Affairs and Forestry, 1st edn. Resource Quality Services, Pretoria
Global Invasive Species Database (GISD) (2005) http://www.issg.org/database. Accessed May 26 2010
Hill MP, Cilliers CJ (1999) A review of the arthropod natural enemies, and factors that influence their efficacy, in the biological control of water hyacinth, Eichhornia crassipes Solms-Laubach (Ponterderiaceae), in South Africa. Afr Entomol Mem 1:103–112
Hulme PE (2006) Beyond control: wider implications for the management of biological invasions. J Appl Ecol 43:835–847
Jones RW (2001) Integrated control of water hyacinth on the Nseleni/Mposa Rivers and Lake Nsezi in KwaZulu-Natal, South Africa. In: Julien MH, Hill MP, Center TD, Jianqing D (eds). Biological and integrated control of water hyacinth, Eichhornia crassipes. Proceedings of the 2nd meeting of the global working group for the biological and integrated control of water hyacinth, Beijing, China, 9-12 October 2000. Australian Centre for International Agricultural Research, Canberra, Australia, pp: 123–129
Jones RW, Weyl OLF, Swartz ER, Hill MP (2013) Using a unified invasion framework to characterize Africa’s first loricariid catfish invasion. Biol Invasions. doi:10.1007/s10530-013-0438-7
Lindegaard C (1994) The role of zoobenthos in energy flow of two shallow lakes. Hydrobiol 275(276):313–322
Longino JT, Coddington JA, Colwell RK (2002) The ant fauna of a tropical rain forest: estimating species richness three different ways. Ecology 83:689–702
Magurran AE (2004) Measuring biological diversity. Blackwell Science Ltd, Oxford
Mangas-Ramirez E, Elias-Gutierrez M (2004) Effect of mechanical removal of water hyacinth (Eichhornia crassipes) on the water quality and biological communities in a Mexican reservoir. J. Aquat Health Manage 7:161–168
Masifwa WF, Twongo T, Denny P (2001) The impact of water hyacinth, Eichhornia crassipes Solms on the abundance and diversity of aquatic macroinvertebrates along the shores of northern lake Victoria, Uganda. Hydrobiol 451:79–88
Midgley JM, Hill MP, Villet MH (2006) The effect of water hyacinth, Eichhornia crassipes Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the new year’s River, South Africa. Afr J Aquat Sci 31:25–30
Mitchell DS (1985) Surface-floating aquatic macrophytes. In: Denny P (ed) The ecology and management of African Wetland vegetation. Junk Publishers, Dordrecht, pp 109–124
Neira C, Levin LA, Grosholz ED (2005) Benthic macrofaunal communities of three sites in San Francisco Bay invaded by hybrid Spartina, with comparison to uninvaded habitats. Mar Ecol Prog Ser 292:111–126
Richardson DM, van Wilgen BW (2004) Invasive alien plants in South Africa: how well do we understand the ecological impacts? S Afr J Sci 100:45–52
Rocha-Ramírez A, Ramírez-Rojas A, Chávez-López R, Alcocer J (2007) Invertebrate assemblages associated with root masses of Eichhornia crassipes Solms-Laubach 1883 in the Alvarado Lagoonal System, Veracruz, Mexico. Aquat Ecol 41:319–333
Rommens W, Maes J, Dekeza N, Inghelbrecht P, Nhiwatiwa T, Holsters E, Ollevier F, Marshall B, Brendonck L (2003) The impact of water hyacinth (Eichhornia crassipes) in a eutrophic subtropical impoundment (Lake Chivero, Zimbabwe). I. Water quality. Arch Hydrobiol 158:373–388
Samadi S, Balzan C, Delay B, Pointier J-P (1997) Local distribution and abundance of thiarid snails in recently colonized rivers from the Caribbean area. Malacol Rev 30:45–52
Schramm HLK, Jirka J, Hoyer MV (1987) Epiphytic macroinvertebrates on dominant macrophytes in two central Florida Lakes. J. Freshwater Ecol 4:151–161
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Thirion C (2000) A new biomonitoring protocol to determine the ecological health of impoundments, using artificial substrates. Afr J Aquat Sci 25:123–133
Toft JD, Simenstad CA, Cordell JR, Grimaldo LF (2003) The effects of introduced water hyacinth on habitat structure, invertebrate assemblages, and fish diets. Estuaries 26:746–758
Toti SD, Coyle FA, Miller JA (2000) A structured inventory of appalachian grass bald and heath bald spider assemblages and a test of species richness estimator performance. J Arachnol 28:329–345
Ultsch GR (1973) The effects of water hyacinth (Eichhornia crassipes) on the microenvironment of aquatic communities. Arch Hydrobiol 72:460–473
Villamagna AM, Murphy BR (2010) Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biol 55:282–298
Wallace JB, Webster JR (1996) The role of macroinvertebrates in stream ecosystem function. Ann Rev Entomol 41:115–139
Acknowledgments
The authors acknowledge financial assistance from the Working for Water programme of the Department of Environmental Affairs, and logistical assistance from Ezemvelo-KwaZulu Natal Wildlife.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Eliska Rejmankova.
Rights and permissions
About this article
Cite this article
Coetzee, J.A., Jones, R.W. & Hill, M.P. Water hyacinth, Eichhornia crassipes (Pontederiaceae), reduces benthic macroinvertebrate diversity in a protected subtropical lake in South Africa. Biodivers Conserv 23, 1319–1330 (2014). https://doi.org/10.1007/s10531-014-0667-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0667-9
Keywords
Profiles
- Julie A. Coetzee View author profile