Abstract
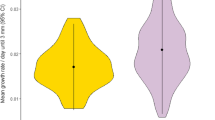
Behavior can be an important determinant of invasion success. In the New Zealand mud snail (Potamopyrgus antipodarum), behavior is influential in predator avoidance and probably plays a role in dispersal. The present study investigated differences in behavior among populations of different asexual clones of this species and compared introduced populations characterized by various levels of invasiveness and New Zealand native clonal populations with respect to rheotactic, geotactic, photokinetic, and dispersal behaviors. There was a significant difference in behavior among populations in all behaviors evaluated. A population of a widespread clone (US1) behaved most differently from the other populations exhibiting differences in all behaviors including a greater propensity to disperse. These results indicate that there is a population and possibly a genotypic effect on behaviors in this freshwater snail, and this variation may help to explain why some clones are more invasive than others.





Similar content being viewed by others
References
Alonso A, Castro-Díez P (2008) What explains the invading success of the aquatic mud snail Potamopyrus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia 614:107–116
Alonso A, Castro-Díez P (2012) The exotic aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca): state of the art of a worldwide invasion. Aquat Sci 74:375–383
Aubry S, Labaune C, Magnin F, Roche P, Kiss L (2006) Active and passive dispersal of an invading land snail in Mediterranean France. J Anim Ecol 75:802–813
Blackburn TM, Cassey P, Lockwood JL (2009) The role of species traits in the establishment success of exotic birds. Glob Change Biol 15:2852–2860
Bowler PA (1991) The rapid spread of the freshwater hydrobiid snail Potamopyrgus antipodarum (Gray) in the Middle Snake River, Southern Idaho. Proc Desert Fish Counc 21:173–182
Dick JTA (2008) Role of behaviour in biological invasions and species distributions; lessons from interactions between the invasive Gammarus pulex and the native G. duebeni (Crustacea: Amphipoda). Contrib Zool 77(2):91–98
Drown DM, Levri EP, Dybdahl MF (2011) Invasive genotypes are opportunistic specialists not general purpose genotypes. Evol Appl 4(1):132–143
Dybdahl MF, Drown DM (2011) The absence of genotypic diversity in a successful parthenogenetic invader. Biol Invasions 13:1663–1672
Fromme AE, Dybdahl MF (2006) Resistance in introduced populations of a freshwater snail to native range parasites. J Evol Biol 19:1948–1955
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506
Haynes A, Taylor BJR, Varley ME (1985) The influence of the mobility of Potamopyrus jenkinsi (Smith, E. A.) (Prosobranchia: Hydrobiidae) on its spread. Arch Hydrobiol 100:479–491
Hazlett BA, Burba A, Gherardi F, Acquistapace P (2003) Invasive species of crayfish use a broader range of predation-risk cues than native species. Biol Invasions 5:223–228
Holway DA, Suarez AV (1999) Animal behavior: an essential component of invasion biology. Trends Ecol Evol 14(8):328–330
Hughes DA (1970) Some factors affecting drift and upstream movements of Gammarus pulex. Ecology 51(2):301–305
Kappes H, Haase P (2012) Slow, but steady: dispersal of freshwater molluscs. Aquat Sci 74:1–14
Kistner EJ, Dybdahl MF (2013) Adaptive responses and invasion: the role of plasticity and evolution in snail shell morphology. Ecol Evol 3(2):424–436
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Leibl AL, Martin LB (2012) Exploratory behavior and stressor hyper-responsiveness facilitate range expansion of an introduced songbird. Proc R Soc [Biol] 279:4375–4381
Lester PJ (2005) Determinants for the successful establishment of exotic ants in New Zealand. Divers Distrib 11:279–288
Levri EP (1998) Perceived predation risk, parasitism, and the foraging behavior of a freshwater snail (Potamopurgus antipodarum). Can J Zool 76:1878–1884
Levri EP (1999) Parasite-induced changes in host behavior of a freshwater snail: manipulation or byproduct of parasitism. Behav Ecol 10(3):234–241
Levri EP, Fisher L (2000) The effect of a trematode parasite (Microphallus sp.) on the response of the freshwater snail, Potamopyrgus antipodarum to light and gravity. Behaviour 137:1141–1151
Levri EP, Jacoby W (2008) The invasive New Zealand mud snail (Potamopyrgus antipodarum) found in streams of the Lake Ontario watershed. J Penn Acad Sci 82(1):7–11
Levri EP, Lively CM (1996) The effects of size, reproductive condition, and parasitism on the foraging behaviour in a freshwater snail, Potamopyrgus antipodarum. Anim Behav 51:891–901
Levri EP, Kelly A, Love E (2007a) The Invasive New Zealand Mud Snail (Potamopyrgus antipodarum) in Lake Erie. J Great Lakes Res 33(1):1–6
Levri EP, Lunnen S, Itle C, Martin T, Kincade B, DeLisser M, Mosquea L (2007b) Parasite-induced alteration of diurnal rhythms in a freshwater snail. J Parasitol 93(2):231–237
Levri EP, Dermott RM, Lunnen SJ, Kelly AA, Ladson T (2008) The distribution of the invasive New Zealand mud snail (Potamopyrgus antipodarum) in Lake Ontario. Aquat Ecosyst Health Manag 11(4):412–421
Levri EP, Colledge E, Bilka R, Smith B (2012a) The distribution of the New Zealand mud snail in streams of the Lake Ontario and Lake Erie watersheds. BioInvasions Rec 1(3):215–219
Levri EP, Dubensky AN, Mears AS, Opiela CA (2012b) Interpopulation variation in predator avoidance behavior of a freshwater snail to the same predator. Can J Zool 90:616–623
Levri EP, Opiela CA, Bilka R (2013) The invasive New Zealand mud snail, (Potamopyrgus antipodarum) not detected in western Lakes Huron and St. Clair. J Penn Acad Sci 87(1):10–15
Levri EP, Krist AC, Bilka R, Dybdahl MF (2014) Phenotypic plasticity of the introduced New Zealand mud snail, Potamopyrgus antipodarum, compared to sympatric native snails. PLoS ONE 9(4):e93985. doi:10.1371/journal.pone.0093985
Lively CM (1987) Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature 328:519–521
Naddafi R, Rudstam LG (2013) Predator-induced behavioural defences in two competitive invasive species: the zebra mussel and the quagga mussel. Anim Behav 86:1275–1284
Neiman M, Hehman G, Miller JT, Logsdon JM Jr, Taylor DR (2010) Accelerated mutation accumulation in asexual lineages of a freshwater snail. Mol Biol Evol 27(4):954–963
Neiman M, Paczesniak D, Soper DM, Baldwin AT, Hehman G (2011) Wide variation in ploidy level and genome size in a New Zealand freshwater snail with coexisting sexual and asexual lineages. Evolution 65(11):3202–3216
Paczesniak D, Jokela J, Larkin K, Neiman M (2013) Discordance between nuclear and mitochondrial genomes in sexual and asexual lineages of the freshwater snail Potampyrgus antipodarum. Mol Ecol 22:4695–4710
Pavlov DS, Mikheev VN, Dgebuadze YY (2006) Behavioral aspects of biological invasion of alien fish species. J Ichthyol 46:S117–S124
Pennuto C, Keppler D (2008) Short-term predator avoidance behavior by invasive and native amphipods in the Great Lakes. Aquat Ecol 42:629–641
Phillips BL, Suarez AV (2012) The role of behavioural variation in the invasion of new areas. In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world. Oxford University Press, Oxford
Phillips BL, Brown GP, Travis JMJ, Shine R (2008) Reid’s paradox revisited: the evolution of dispersal in range-shifting populations. Am Nat 172:S34–S48
Pintor LM, Sih A (2009) Differences in growth and foraging behavior of native and introduced populations of an invasive crayfish. Biol Invasions 11:1895–1902
Proctor T, Kerans B, Clancey P, Ryce E, Dybdahl M, Gustafson D, Hall R, Pickett F, Richards D, Waldeck RD, Chapman J, Wiltshire RH, Becker D, Anderson M, Pitman B, Lassuy D, Heimowitz P, Dwyer P, Levri EP (2007) National management and control plan for the New Zealand Mudsnail (Potamopyrgus antipodarum). www.anstaskforce.gov/Documents/NZMS_MgmtControl_Final.pdf
Rehage JS, Sih A (2004) Dispersal Behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol Invasions 6:379–391
Rehage JS, Barnett BK, Sih A (2005a) Behavioral responses to a novel predator and competitor of invasive mosquitofish and their non-invasive relatives (Gambusia sp.). Behav Ecol Sociobiol 57:256–266
Rehage JS, Barnett BK, Sih A (2005b) Foraging behaviour and invasiveness: do invasive Gambusia exhibit higher feeding rates and broader diets than their noninvasive relatives? Ecol Freshw Fish 14:352–360
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Sepulveda AJ, Marczak LB (2012) Active dispersal of an aquatic invader determined by resource and flow conditions. Biol Invasions 14:1201–1209
Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63:495–502
Sol D, Bacher S, Reader SM, Lefebvre L (2008) Brain size predicts the success of mammal species introduced to novel environments. Am Nat 172:S63–S71
Suarez AV, Holway DA, Ward PS (2005) The role of opportunity in the unintentional introduction of nonnative ants. Proc Natl Acad Sci USA 102:17032–17035
USGS (2013) New Zealand Mudsnail, Potamopyrgus antipodarum. United States Geological Survey. http://fl.biology.usgs.gov/Nonindigenous_Species/New_Zealand_Mudsnail/new_zealand_mudsnail.html. Accessed 25 May 2014
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press, San Diego
Winterbourn M (1970) The New Zealand species of Potamopyrgus (Gastropoda: Hydrobidae). Malacalogia 10(2):283–321
Zaranko DT, Farara DG, Thompson FG (1997) Another exotic mollusk in the Laurentian Great Lakes: the New Zealand native Potamopyrgus antipodarum (Gray 1843) (Gastropoda, Hydrobiidae). Can J Fish Aquat Sci 54:809–814
Acknowledgments
We thank Frank Menaquale, Sarah Landis, Brittany Smith, Megan Radyk, Elizabeth Metz, and Christina Lehman for assistance in the lab, Mark Oswalt for logistical support, and Maurine Neiman (New Zealand) and Mark Dybdahl (Idaho, Wyoming) for donating many of the clones. We also thank Maureen Levri, Stephanie Tanner, two anonymous reviewers, and an anonymous editor for comments on earlier versions of the manuscript and Laura Palmer for assistance with figures. This work was funded by grants from Penn State – Altoona and the Penn State – Altoona Division of Mathematics and Natural Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levri, E.P., Clark, T.J. Behavior in invasive New Zealand mud snails (Potamopyrgus antipodarum) is related to source population. Biol Invasions 17, 497–506 (2015). https://doi.org/10.1007/s10530-014-0746-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0746-6