Abstract
Purpose
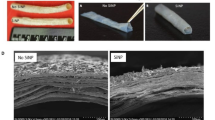
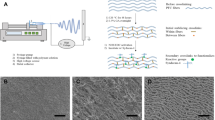
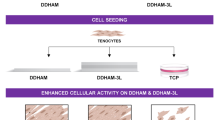
The limited availability of autologous vessels for vascular bypass surgeries is a major roadblock to treating severe cardiovascular diseases. Based on this clinical priority, our group has developed a novel engineered vascular graft by rolling human amniotic membranes into multilayered extracellular matrixes (ECM). When treated with silica nanoparticles (SiNP), these rolled scaffolds showed a significant improvement in their structural and mechanical properties, matching those from gold standard autologous grafts. However, it remained to be determined how cells respond to SiNP-treated materials. As a first step toward understanding the biocompatibility of SiNP-dosed biomaterials, we aimed to assess how endothelial cells and blood components interact with SiNP-treated ECM scaffolds.
Methods
To test this, we used established in vitro assays to study SiNP and SiNP-treated scaffolds’ cyto and hemocompatibility.
Results
Our results showed that SiNP effects on cells were concentration-dependent with no adverse effects observed up to 10 μg/ml of SiNP, with higher concentrations inducing cytotoxic and hemolytic responses. The SiNP also enhanced the scaffold’s hydrophobicity state, a feature known to inhibit platelet and immune cell adhesion. Accordingly, SiNP-treated scaffolds were also shown to support endothelial cell growth while preventing platelet and leukocyte adhesion.
Conclusion
Our findings suggest that the addition of SiNP to human amniotic membrane extracellular matrixes improves the cyto- and hemocompatibility of rolled scaffolds and highlights this strategy as a robust mechanism to stabilize layered collagen scaffolds for vascular tissue regeneration.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Allen CL, Clare G, Stewart EA et al (2013) Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0078441
Ambler GK, Twine CP (2018) Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001487.pub3
Amensag S, McFetridge PS (2012) Rolling the human amnion to engineer laminated vascular tissues. Tissue Eng Part C 18:903–912. https://doi.org/10.1089/ten.tec.2012.0119
Amensag S, McFetridge PS (2014) Tuning scaffold mechanics by laminating native extracellular matrix membranes and effects on early cellular remodeling. J Biomed Mater Res Part A 102:1325–1333. https://doi.org/10.1002/jbm.a.34791
Amensag S, Goldberg LA, O’Malley KA et al (2017) Pilot assessment of a human extracellular matrix-based vascular graft in a rabbit model. J Vasc Surg 65:839. https://doi.org/10.1016/j.jvs.2016.02.046
Arasteh S, Kazemnejad S, Khanjani S et al (2016) Fabrication and characterization of nano-fibrous bilayer composite for skin regeneration application. Methods 99:3–12. https://doi.org/10.1016/j.ymeth.2015.08.017
Bota PCS, Collie AMB, Puolakkainen P et al (2010) Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res Part A 95:649–657. https://doi.org/10.1002/jbm.a.32893
Boura C, Menu P, Payan E et al (2003) Endothelial cells grown on thin polyelectrolyte mutlilayered films: an evaluation of a new versatile surface modification. Biomaterials 24:3521–3530. https://doi.org/10.1016/S0142-9612(03)00214-X
Caines AEB, Massad MG, Kpodonu J et al (2004) Outcomes of coronary artery bypass grafting versus percutaneous coronary intervention and medical therapy for multivessel disease with and without left ventricular dysfunction. Cardiology 101:21–28. https://doi.org/10.1159/000075982
Cardozo LAM, Rouw DB, Ambrose LR et al (2004) The neutrophil: the unnoticed threat in xenotransplantation? Transplantation 78:1721–1728. https://doi.org/10.1097/01.TP.0000147341.40485.B4
Carrabba M, Madeddu P (2018) Current strategies for the manufacture of small size tissue engineering vascular grafts. Front Bioeng Biotechnol 6:41
Casa LDC, Deaton DH, Ku DN (2015) Role of high shear rate in thrombosis. Mosby, Maryland Heights
Center for Disease Control and Prevention, Underlying Cause of Death 1999-2020. (2020) [cited 11/01/2023]; Available from: https://wonder.cdc.gov/wonder/help/ucd.html
Chen J, López JA (2005) Interactions of platelets with subendothelium and endothelium. Microcirculation 12(3):235–246
Cheryl F, Chen T-C, Li X (2012) Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Br 103
Coller BS, Shattil SJ (2008) The GPIIb/IIIa (integrin άIIbΒ3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Am Soc Hematol 112:3011–3025
Dardik H, Wengerter K, Qin F et al (2002) Comparative decades of experience with glutaraldehyde-tanned human umbilical cord vein graft for lower limb revascularization: an analysis of 1275 cases. J Vasc Surg 35:64–71. https://doi.org/10.1067/mva.2002.121053
Fukuzaki S, Urano H, Nagata K (1996) Adsorption of bovine serum albumin onto metal oxide surfaces. J Ferment Bioeng 81:163–167. https://doi.org/10.1016/0922-338X(96)87596-9
Furukawa KS, Nakamura K, Onimura Y et al (2010) Quantitative analysis of human platelet adhesions under a small-scale flow device. Artif Organs 34:295–300. https://doi.org/10.1111/j.1525-1594.2009.00862.x
Gonçalves MC (2018) Sol-gel silica nanoparticles in medicine: a natural choice. Design, synthesis and products. Molecules. https://doi.org/10.3390/molecules23082021
Heron M (2021) Deaths: leading causes for 2019. Natl Vital Stat Rep 70:1–114
Hopkinson A, McIntosh RS, Tighe PJ et al (2006) Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF-beta content. Invest Ophthalmol vis Sci 47:4316–4322. https://doi.org/10.1167/IOVS.05-1415
Jaffer IH, Weitz JI (2019) The blood compatibility challenge. Part 1: blood-contacting medical devices: the scope of the problem. Acta Biomater 94:2–10
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745–2756. https://doi.org/10.1172/JCI107470
Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI (2015) Medical device-induced thrombosis: what causes it and how can we prevent it? Wiley, New York
Janjua TI, Cao Y, Yu C, Popat A (2021) Clinical translation of silica nanoparticles. Nat Rev Mater 6:1072–1074. https://doi.org/10.1038/s41578-021-00385-x
Kakavand M, Yazdanpanah G, Ahmadiani A, Niknejad H (2017) Blood compatibility of human amniotic membrane compared with heparin-coated ePTFE for vascular tissue engineering. J Tissue Eng Regen Med 11:1701–1709. https://doi.org/10.1002/term.2064
Langer R (2000) Biomaterials: status, challenges, and perspectives. AIChE J 46:1286–1289
L’heureux N, Pâquet S, Labbé R et al (1998) A completely biological tissue-engineered human blood vessel. FASEB J 12:47–56. https://doi.org/10.1096/fasebj.12.1.47
Mallis P, Kostakis A, Stavropoulos-Giokas C, Michalopoulos E (2020) Future perspectives in small-diameter vascular graft engineering. Bioengineering 7:1–40. https://doi.org/10.3390/bioengineering7040160
Mamede AC, Carvalho MJ, Abrantes AM et al (2012) Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349:447–458. https://doi.org/10.1007/s00441-012-1424-6
Meddahi-Pellé A, Legrand A, Marcellan A et al (2014) Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem 53:6369–6373. https://doi.org/10.1002/anie.201401043
Menter DG, Kopetz S, Hawk E, et al (2017) Platelet “first responders” in wound response, cancer, and metastasis. NIH Public Access
Mrówczyński W, Mugnai D, De Valence S et al (2014) Porcine carotid artery replacement with biodegradable electrospun poly-e-caprolactone vascular prosthesis. J Vasc Surg 59:210–219. https://doi.org/10.1016/j.jvs.2013.03.004
Ozawa T, Mickle DAG, Weisel RD et al (2002) Optimal biomaterial for creation of autologous cardiac grafts. Circulation 106:I176–I182. https://doi.org/10.1161/01.cir.0000032901.55215.cc
Pashneh-Tala S, MacNeil S, Claeyssens F (2016) The tissue-engineered vascular graft—past, present, and future. Tissue Eng 22:68–100. https://doi.org/10.1089/ten.teb.2015.0100
Russell MW, Huse DM, Drowns S et al (1998) Direct medical costs of coronary artery disease in the United States. Am J Cardiol 81:1110–1115. https://doi.org/10.1016/S0002-9149(98)00136-2
Schmidt SP, Bowlin G, Schmidt SP, Bowlin GL (1999) Endothelial cell seeding: a review. In: Zilla P, Greisler HP (eds) Tissue engineering of vascular prosthetic grafts. R.G. Lanes Company, Austin, pp 61–67
Shabalovskaya SA, Siegismund D, Heurich E, Rettenmayr M (2013) Evaluation of wettability and surface energy of native Nitinol surfaces in relation to hemocompatibility. Mater Sci Eng C 33:127–132. https://doi.org/10.1016/j.msec.2012.08.018
Tiwari A, Salacinski H, Seifalian AM et al (2002) New prostheses for use in bypass grafts with special emphasis on polyurethanes. Cardiovasc Surg 10:191–197. https://doi.org/10.1016/S0967-2109(02)00004-2
Tsai WB, Grunkemeier JM, McFarland CD, Horbett TA (2002) Platelet adhesion to polystyrene-based surfaces preadsorbed with plasmas selectively depleted in fibrinogen, fibronectin, vitronectin, or von Willebrand’s factor. J Biomed Mater Res 60:348–359. https://doi.org/10.1002/jbm.10048
Tukiainen E, Kallio M, Lepäntalo M (2006) Advanced leg salvage of the critically ischemic leg with major tissue loss by vascular and plastic surgeon teamwork: long-term outcome. Ann Surg 244:949–957. https://doi.org/10.1097/01.sla.0000247985.45541.e8
Uzarski JS, Van De Walle AB, McFetridge PS (2014) In vitro method for real-time, direct observation of cell-vascular graft interactions under simulated blood flow. Tissue Eng Part C 20:116–128. https://doi.org/10.1089/ten.tec.2012.0771
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MSCC (2021) Heart disease and stroke statistics-2021 update a report from the American Heart Association. Circulation 135:e146–e603
Walgenbach KJ, Bannasch H, Kalthoff S, Rubin JP (2012) Randomized, prospective study of TissuGlu® surgical adhesive in the management of wound drainage following abdominoplasty. Aesthetic Plast Surg 36:491–496. https://doi.org/10.1007/S00266-011-9844-3
Wang X, Lin P, Yao Q, Chen C (2007) Development of small-diameter vascular grafts. World J Surg. https://doi.org/10.1007/s00268-006-0731-z
Wu XH, Liew YK, Mai CW, Then YY (2021) Potential of superhydrophobic surface for blood-contacting medical devices. Int J Mol Sci 22:3341
Zhang H, Dunphy DR, Jiang X et al (2012) Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs pyrolytic. J Am Chem Soc 134:15790–15804. https://doi.org/10.1021/ja304907c
Zhao Y, Sun X, Zhang G et al (2011) Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano 5:1366–1375. https://doi.org/10.1021/nn103077k
Zhuang Y, Zhang C, Cheng M et al (2021) Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact Mater 6:1791–1809
Acknowledgements
Special thanks to Kimberly Backer-Kelley of the Interdisciplinary Center for Biotechnology Research for her support at the Electron Microscopy core lab. UF Health Shands Labor & Delivery unit. Dr. Dara Wakefield and Louis Kauo of the UF Health Shands Department of Pathology.
Funding
Financial support provided by the National Institute of Health (NIH R01 HL088207).
Author information
Authors and Affiliations
Contributions
LAG: Performed experiments, analyzed the data and wrote the paper; HDZ: analyzed the data and wrote the paper; CMF: performed experiments, wrote the paper; PMF: Supervised the study, analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
Placental tissues were obtained from the Labor & Delivery department at UF Health Shands Hospital at the University of Florida (Gainesville, FL, IRB Approval #64-2010).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goldberg, L.A., Zomer, H.D., McFetridge, C. et al. Silica nanoparticles enhance the cyto- and hemocompatibility of a multilayered extracellular matrix scaffold for vascular tissue regeneration. Biotechnol Lett 46, 249–261 (2024). https://doi.org/10.1007/s10529-023-03459-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-023-03459-8