Abstract
Objectives
Shellfish waste is a primary source for making N-acetyl-d-glucosamine. Thus, establishing a high-efficiency and low-cost bioconversion method to produce N-acetyl-d-glucosamine directly from shellfish waste was promising.
Results
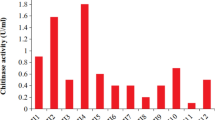
A mutant C81 was obtained from Chitinolyticbacter meiyuanensis SYBC-H1 via 60Co-γ irradiation. This mutant C81 showed the highest chitinase activity of 9.8 U/mL that was 85% higher than the parent strain. The mutant C81 exhibted improved antioxidant activities, including total antioxidant capacity, superoxide radical ability, and hydroxyl radical scavenging ability, compared to that of the parent strain. Four out of nine organic solvents increased the chitinase activity by 1.9%, 6.8%, 11.7%, and 15.8%, corresponding to methylbenzene, n-heptane, petroleum ether, and n-hexane, respectively. The biphase system composed of aqueous and hexane presented a five-fold reduction of cell viability compared to the control. Using a continuous fermentation bioconversion process, 4.2 g/L GlcNAc was produced from crayfish shell powder with a yield of 80% of the chitin content.
Conclusions
This study demonstrated that the mutant C81 is suitable for converting crayfish shell powder into GlcNAc in an aqueous-organic system.




Similar content being viewed by others
References
Anil Kumar PK, Suresh PV (2014) Biodegradation of shrimp biowaste by marine Exiguobacterium sp. CFR26M and concomitant production of extracellular protease and antioxidant materials: production and process optimization by response surface methodology. Mar Biotechnol (NY) 16:202–218
Annamalai N, Veeramuthu Rajeswari M, Vijayalakshmi S, Balasubramanian T (2011) Purification and characterization of chitinase from Alcaligenes faecalis AU02 by utilizing marine wastes and its antioxidant activity. Ann Microbiol 61:801–807
Chen GQ, Jiang XR (2017) Next generation industrial biotechnology based on extremophilic bacteria. Curr Opin Biotechnol 50:94–100
Chung D, Baek K, Bae SS, Jung J (2019) Identification and characterization of a marine-derived chitinolytic fungus, Acremonium sp. YS2-2. J Microbiol 57:372–380
Ding Q, Luo QL, Zhou J, Chen XL, Liu LM (2018) Enhancing l-malate production of Aspergillus oryzae FMME218-37 by improving inorganic nitrogen utilization. Appl Microbiol Biotechnol 102:8739–8751
Dun Y, Li Y, Xu J, Hu Y, Zhang C, Liang Y, Zhao S (2019) Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int J Biol Macromol 123:420–426
Gao C, Zhang A, Chen KQ, Hao ZK, Tong JM, Ouyang PK (2015) Characterization of extracellular chitinase from Chitinibacter sp GC72 and its application in GlcNAc production from crayfish shell enzymatic degradation. Biochem Eng J 97:59–64
Hamed I, Özogul F, Regenstein JM (2016) Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Technol 48:40–50
Hao Z, Wu H, Yang M, Chen J, Xi L, Zhao W, Yu J, Liu J, Liao X, Huang Q (2016) Cloning, expression and 3D structure prediction of chitinase from Chitinolyticbacter meiyuanensis SYBC-H1. Int J Mol Sci 17:825
Jiang Y, Guo D, Lu J, Durre P, Dong W, Yan W, Zhang W, Ma J, Jiang M, Xin F (2018) Consolidated bioprocessing of butanol production from xylan by a thermophilic and butanologenic Thermoanaerobacterium sp. M5. Biotechnol Biofuels 11:89
Kidibule PE, Santos-Moriano P, Plou FJ, Fernandez-Lobato M (2020) Endo-chitinase Chit33 specificity on different chitinolytic materials allows the production of unexplored chitooligosaccharides with antioxidant activity. Biotechnol Rep (amst) 27:e00500
Lee HJ, Lee YS, Choi YL (2018) Cloning, purification, and characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221. Biotechnol Biofuels 11:303
Liu LM, Xu QL, Li Y, Shi ZP, Zhu Y, Du GC, Chen J (2007) Enhancement of pyruvate osmotic-tolerant mutant production by of Torulopsis glabrata. Biotechnol Bioeng 97:825–832
Mathew GM, Mathew DC, Sukumaran RK, Sindhu R, Huang CC, Binod P, Sirohi R, Kim SH, Pandey A (2020) Sustainable and eco-friendly strategies for shrimp shell valorization. Environ Pollut 267:115656
Nawani NN, Prakash D, Kapadnis BP (2010) Extraction, purification and characterization of an antioxidant from marine waste using protease and chitinase cocktail. World J Microbiol Biotechnol 26:1509–1517
Robles-Arias MA, Garcia-Garibay M, Alatorre-Santamaria S, Tello-Solis SR, Guzman-Rodriguez F, Gomez-Ruiz L, Rodriguez-Serrano G, Cruz-Guerrero AE (2021) Improvement of fucosylated oligosaccharides synthesis by alpha-L-fucosidase from Thermotoga maritima in water-organic cosolvent reaction system. Appl Biochem Biotechnol 193:3553–3569
Sedaghat F, Yousefzadi M, Toiserkani H, Najafipour S (2017) Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: an alternative procedure for chemical extraction of chitin and chitosan. Int J Biol Macromol 104:883–888
Subramanian K, Sadaiappan B, Aruni W, Kumarappan A, Thirunavukarasu R, Srinivasan GP, Bharathi S, Nainangu P, Renuga PS, Elamaran A, Balaraman D, Subramanian M (2020) Bioconversion of chitin and concomitant production of chitinase and N-acetylglucosamine by novel Achromobacter xylosoxidans isolated from shrimp waste disposal area. Sci Rep 10:11898
Suryawanshi N, Eswari JS (2021) Chitin from seafood waste: particle swarm optimization and neural network study for the improved chitinase production. J Chem Technol Biotechnol 97:509–519
Wang YT, Wu PL (2020) Gene cloning, characterization, and molecular simulations of a novel recombinant chitinase from Chitinibacter Tainanensis CT01 appropriate for chitin enzymatic hydrolysis. Polymers (basel) 12:1648
Wang SL, Lin CL, Liang TW, Liu KC, Kuo YH (2009) Conversion of squid pen by Serratia ureilytica for the production of enzymes and antioxidants. Bioresour Technol 100:316–323
Wang D, Li A, Han H, Liu T, Yang Q (2018) A potent chitinase from Bacillus subtilis for the efficient bioconversion of chitin-containing wastes. Int J Biol Macromol 116:863–868
Wei G, Zhang A, Chen K, Ouyang P (2017) Enzymatic production of N-acetyl-d-glucosamine from crayfish shell wastes pretreated via high pressure homogenization. Carbohydr Polym 171:236–241
Xu P, Wu X-L, Guo X-X, Tang J, Zong M-H, Lou W-Y (2018) Double-chitinase hydrolysis of crab shell chitin pretreated by Ionic liquid to generate chito-oligosaccharide. Acs Sustain Chem Eng 7:1683–1691
Yadav M, Goswami P, Paritosh K, Kumar M, Pareek N, Vivekanand V (2019) Seafood waste: a source for preparation of commercially employable chitin/chitosan materials. Bioresour Bioprocess 6:8
Yahiaoui M, Laribi-Habchi H, Bouacem K, Asmani KL, Mechri S, Harir M, Bendif H, Aissani-El Fertas R, Jaouadi B (2019) Purification and biochemical characterization of a new organic solvent-tolerant chitinase from Paenibacillus timonensis strain LK-DZ15 isolated from the Djurdjura Mountains in Kabylia, Algeria. Carbohydr Res 483:107747
Zhang H, Jin Y, Deng Y, Wang D, Zhao Y (2012) Production of chitin from shrimp shell powders using Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydr Res 362:13–20
Zhang A, Gao C, Wang J, Chen K, Ouyang P (2016) An efficient enzymatic production of N-acetyl-d-glucosamine from crude chitin powders. Green Chem 18:2147–2154
Zhang Q, Wang L, Liu S, Li Y (2021) Establishment of successive co-fermentation by Bacillus subtilis and Acetobacter pasteurianus for extracting chitin from shrimp shells. Carbohydr Polym 258:117720
Acknowledgements
This work was supported by the Science and Technology Project of Taizhou (1801gy24, 21gyb27), The Vertical Project Transmuted from the Horizontal Project of Taizhou Vocational and Technical college (2020HGZ01), the Doctoral Foundation Project of Taizhou Vocational and Technical College (2021BS02, 2016BSH01). Zhejiang Province Key Research and Development Plan (2021C03190), Scientific Research Foundation of Zhejiang A&F University (2034020081).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hao, Zk., Li, Js., Wang, Dh. et al. Efficient production of GlcNAc in an aqueous-organic system with a Chitinolyticbacter meiyuanensis SYBC-H1 mutant. Biotechnol Lett 44, 623–633 (2022). https://doi.org/10.1007/s10529-022-03248-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03248-9