Abstract
Objectives
To biotransform rutin into isoquercitrin.
Results
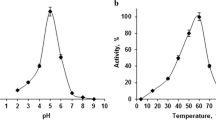
A α-l-rhamnosidase from Bifidobacterium breve was produced by using Escherichia coli BL21 for biotransformation of rutin to isoquercitrin. The enzyme was purified by Ni2+-NTA chromatography to yield a soluble protein with a specific activity of 56 U protein mg−1. The maximum enzyme activities were at pH 6.5, 55 °C, 20 mM rutin, and 1.2 U enzyme ml−1. Under optimal conditions, the half-life of the enzyme was 96 h. The K m and V max values were 2.2 mM, 56.4 μmol mg−1 min−1 and 2.1 mM, 57.5 μmol mg−1 min−1 using pNP-Rha and rutin as substrates, respectively. The kinetic behavior indicated that the recombinant α-l-rhamnosidase has good catalytic performance for producing isoquercitrin. 20 mM rutin was biotransformed into 18.25 and 19.87 mM isoquercitrin after 60 and 240 min.
Conclusion
The specific biotransformation of rutin to isoquercitrin using recombinant α-l-rhamnosidase from B. breve is a feasible method for use in industrial processes.






Similar content being viewed by others
References
Arts IC, Sesink AL, Faassen-Peters M, Hollman PC (2004) The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br J Nutr 91:841–847
Awatsuhara R, Harada K, Maeda T, Nomura T, Nagao K (2010) Antioxidative activity of the buckwheat polyphenol rutin in combination with ovalbumin. Mol Med Rep 3:121–125
Beekwilder J, Marcozzi D, Vecchi S, de Vos R, Janssen P, Francke C, van Hylckama Vlieg J, Hall RD (2009) Characterization of rhamnosidases from Lactobacillus plantarum and Lactobacillus acidophilus. Appl Environ Microbiol 75:3447–3454
Chua LS (2013) A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol 150:805–817
De Winter K, Simcikova D, Schalck B, Weignerova L, Pelantova H, Soetaert W, Desmet T, Kren V (2013) Chemoenzymatic synthesis of alpha-l-rhamnosides using recombinant alpha-l-rhamnosidase from Aspergillus terreus. Bioresour Technol 147:640–644
Hashimoto W, Nankai H, Sato N, Kawai S, Murata K (1999) Characterization of alpha-l-rhamnosidase of Bacillus sp. GL1 responsible for the complete depolymerization of gellan. Arch Biochem Biophys 368:56–60
Koseki T, Mese Y, Nishibori N, Masaki K, Fujii T, Handa T, Yamane Y, Shiono Y, Murayama T, Iefuji H (2008) Characterization of an alpha-l-rhamnosidase from Aspergillus kawachii and its gene. Appl Microbiol Biotechnol 80:1007–1013
Manzanares P, de Graaff LH, Visser J (1997) Purification and characterization of an alpha-l-rhamnosidase from Aspergillus niger. FEMS Microbiol Lett 157:279–283
Manzanares P, Orejas M, Gil JV, De Graaff LH, Visser J, Ramon D (2003) Construction of a genetically modified wine yeast strain expressing the Aspergillus aculeatus rhaA gene, encoding an alpha-l-rhamnosidase of enological interest. Appl Environ Microbiol 69:7558–7562
Paulke A, Eckert GP, Schubert-Zsilavecz M, Wurglics M (2012) Isoquercitrin provides better bioavailability than quercetin: comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie 67:991–996
Rojas NL, Voget CE, Hours RA, Cavalitto SF (2011) Purification and characterization of a novel alkaline alpha-l-rhamnosidase produced by Acrostalagmus luteo albus. J Ind Microbiol Biotechnol 38:1515–1522
Spagna G, Barbagallo RN, Martino A, Pifferi PG (2000) A simple method for purifying glycosidases: alpha-l-rhamnopyranosidase from Aspergillus niger to increase the aroma of Moscato wine. Enz Microb Technol 27:522–530
Valentova K, Vrba J, Bancirova M, Ulrichova J, Kren V (2014) Isoquercitrin: pharmacology, toxicology, and metabolism. Food Chem Toxicol 68:267–282
Yadav V, Yadav PK, Yadav S, Yadav KDS (2010) α-l-Rhamnosidase: a review. Process Biochem 45:1226–1235
Yanai T, Sato M (2000) Purification and characterization of an alpha-l-rhamnosidase from Pichia angusta X349. Biosci Biotechnol Biochem 64:2179–2185
You HJ, Ahn HJ, Ji GE (2010) Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J Agric Food Chem 58:10886–10892
Acknowledgments
The study was supported by the Natural Science Foundation of Hunan Province (11JJ6009), the Scientific Research Fund of Hunan Provincial Education Department (11C0329) and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (201411342004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, R., Zhang, BL., Xie, T. et al. Biotransformation of rutin to isoquercitrin using recombinant α-l-rhamnosidase from Bifidobacterium breve . Biotechnol Lett 37, 1257–1264 (2015). https://doi.org/10.1007/s10529-015-1792-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1792-6