Abstract
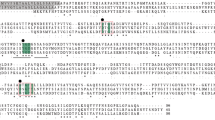
Aprotinin, the most studied serine proteinase inhibitor, was isolated from porcine lung for the first time. The purified porcine aprotinin had an Mr value of ∼7 kDa. It cross-reacted with polyclonal serum anti-commercial aprotinin. About 1 μg porcine aprotinin inhibited 6 μg trypsin whereas 1 μg commercial soybean inhibitor inhibited only 1 μg trypsin. The aprotinin gene was also isolated from porcine lung: the deduced amino acid sequence showed 74% identity to bovine aprotinin.




Similar content being viewed by others
References
Businaro R, Fioretti E, Fumagalli L, Citro G, Renzis G, Ascoli F (1988) Cellular localization of bovine pancreatic trypsin inhibitor and related molecular forms in bovine lung. Histochem J 20:187–193
Foot NJ, Orgeig S, Daniels CB (2006) The evolution of a physiological system: the pulmonary surfactant system in diving mammals. Respir Physiol Neurobiol 154:118–138
Fritz H, Wunderer G (1983) Biochemistry and applications of aprotinin, the kallikrein inhibitor from bovine organs. Arzneim-Forsch/Drug Res 33:479–494
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Ikekita M, Jones CS, Kamo M, Tsugita A, Kizuki K, Moriya H (1992) Purification and characterization of the major cationic kallikrein inhibitor in bovine pituitary gland. Protein Seq Data Anal 5:7–11
Kassell B (1970) Bovine trypsin-kallikrein inhibitor (Kunitz-inhibitor, basic pancreatic trypsin inhibitor, polyvalent inhibitor from bovine organs). Methods Enzymol 19:844–852
Kingston IB, Anderson S (1986) Sequences encoding two trypsin inhibitors occur in strikingly similar genomic environments. Biochem J 233:443–450
Kubrusly FS, Iourtov D, Leme E, Raw I (2004) Pulmonary surfactant protein A isolation as a by-product of porcine pulmonary surfactant production. Biotechnol Appl Biochem 40:173–179
Kubrusly FS, Netto SL, Iourtov D, Raw I, Araujo PS (2000) A natural pig lung surfactant. Biotechnol Lett 22:1251–1253
Laskowski M Jr, Kato I (1980) Proteins inhibitors of Proteinases. Ann Rev Biochem 49:593–626
Laskowski M Jr, Kato I, Kohr WJ, Park SJ, Tashiro M, Whatley HE (1987) Positive Darwinian selection in evolution of protein inhibitors of serine proteinases. Cold Spring Harb Symp Quant Biol 52:545–553
Le QL, Katunuma N (2004) Detection of protease inhibitors by a reverse zymography method, performed in a tris(hydroxymethyl)aminomethane-Tricine buffer system. Anal Biochem 324:237–240
Royston D, van Haaften N, De Vooght P (2007) Aprotinin; friend or foe? A review of recent medical literature. Eur J Anaesthesiol 24:6–14
Yang L, Resnick MI, Marengo SR (2005) A simple procedure for isolating microgram quantities of biologically active bikunin from human urine. Br J Urol 96:647–653
Acknowledgments
The authors are in debt to Mr. Leo Setsuo Kobashi for his technical assistance. This work was supported by grants and scholarships from Brazilian agencies: CNPq, FAPESP, Capes/MEC and by Butantan Foundation and Sadia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Cássia Dias, S., Sakauchi, D., Abreu, P.A.E. et al. Purification and characterization of aprotinin from porcine lungs. Biotechnol Lett 30, 807–812 (2008). https://doi.org/10.1007/s10529-007-9622-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9622-0