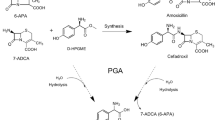
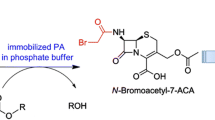
Abstract
A new approach has been developed for the production of enantiomerically pure (S)-β-phenylalanine (S-BPA) and (R)-β-phenylalanine in aqueous medium based on enantioselective acylation and hydrolysis properties of penicillin G acylase from Escherichia coli. The acylation reaction was highly preferential for the acylation of (R)-BPA to form N-phenylacetyl-(R)-BPA using phenylacetamide as an acyl donor, which was separated and then hydrolyzed to (R)-BPA by the same enzyme at pH 7.5. The optimal acylation reaction was at pH 10, 25°C with a 2:1 molar ratio of phenylacetamide to BPA, 8 IU ml−1 enzyme and 150 mM BPA. These resulted in a conversion of about 50% BPA; enantiomeric excess of (S)-BPA and (R)-BPA separated were 98 and 99%, respectively.





Similar content being viewed by others
References
Bruggin K, Roos EC, Devroom E (1998) Penicillin acylase in the industrial production of β-lactam antibiotics. Org Process Res Dev 2:128–133
Chilov GG, Moody HM, Boesten WHJ, Švedas VK (2003) Resolution of (R,S)-phenylglycinonitrile by penicillin acylase-catalyzed acylation in aqueous medium. Tetrahedron Asymmetry 14:2613–2617
Cohen SG, Weinstein SY (1964) Hydrolysis of d(-)-ethyl β-phenyl-β-hydroxypropionate and d(-)-ethyl β-phenyl-β-acetamidopropionate by α-chymotrypsin. J Am Chem Soc 86:725–728
Fadnavis NW, Sharfuddin M, Vadivel SK (1999) Resolution of racemic 2-amino-1-butanol with immobilized penicillin G acylase. Tetrahedron Asymmetry 10:4495–4500
Faulconbridge SJ, Holt KE, Sevillano LG, Lock CJ, Tiffin PD, Tremayne N, Winter S (2000) Preparation of enantiomerically enriched aromatic β-amino acids via enzymatic resolution. Tetrahedron Lett 41:2679–2681
Groeger H, Trauthwein H, Buchholz S, Drauz K, Sacherer C, Godfrin S, Werner H (2004) The first aminoacylase-catalyzed enantioselective synthesis of aromatic β-amino acids. Org Biomol Chem 2:1977–1978
Guranda DT, van Langen LM, van Rantwijk F, Sheldon RA, Švedas VK (2001) Highly efficient and enantioselective enzymatic acylation of amines in aqueous medium. Tetrahedron Asymmetry 12:1645–1650
Khimiuk AY, Korennykh AV, van Langen LM, van Rantwijk F (2003) Penicillin acylase-catalyzed peptide synthesis in aqueous medium: a chemoenzymatic route to stereoisomerically pure diketopiperazines. Tetrahedron Asymmetry 14:3123–3128
Morita H, Nagashima S, Takeya K, Itokawa H, Iitaka Y (1995) Structures and conformation of antitumor cyclic pentapeptides, astin A, B and C, from Aster tataricus. Tetrahedron 51:1121–1132
Nagano Y, Ikedo K, Fujishima A, Izawa M, Tsubotani S, Nishimura O, Fujino M (2001) Pyloricidins, novel anti-Helicobacter pylori antibiotics produced by Bacillus sp. J Antibiot 54:934–947
Podlech J (2005) Preparation of enantiopure β-amino acids by homologation of α-amino acids. In: Juaristi E, Soloshonok VA (eds) Enantioselective synthesis of β-amino acids, 2nd edn. Wiley-VCH, New York, pp 93–106
Preiml M, Hillmayer K, Klempier N (2003) A new approach to β-amino acids: biotransformation of N-protected β-amino nitriles. Tetrahedron Lett 44:5057–5059
Shewale JG, Kumar KK, Ambekar GR (1987) Evaluation of determination 6-aminopenicillanic acid by p-dimethylminobenzaldehyde. Biotechnol Technol 1:69–72
Shewale JG, Deshpande BS, Sudhakaran VK, Ambedkar SS (1992) Penicillin acylase: applications and potentials. Process Biochem 27:131–143
Sivakumar AV, Babu GS, Bhat SV (2001) Asymmetric synthesis of β-amino acids through application of chiral sulfoxide. Tetrahedron Asymmetry 12:1095–1099
Soloshonok VA, Fokina NA, Rybakova AV, Shishkina IP, Galushko SV, Sorochinsky AE, Kukhar VP, Savchenko MV, Švedas VK (1995) Biocatalytic approach to enantiomerically pure β-amino acids. Tetrahedron Asymmetry 6:1601–1610
Spiteller P, von Nussbaum F (2005) β-amino acids in natural products. In: Juaristi E, Soloshonok VA (eds) Enantioselective synthesis of β-amino acids, 2nd edn. Wiley-VCH, New York, pp 19–93
Acknowledgements
This work was supported by the Program for New Century Excellent Talents in University (project No.: NCET-04-0411).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D., Cheng, S., Wei, D. et al. Production of enantiomerically pure (S)-β-phenylalanine and (R)-β-phenylalanine by penicillin G acylase from Escherichia coli in aqueous medium. Biotechnol Lett 29, 1825–1830 (2007). https://doi.org/10.1007/s10529-007-9480-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9480-9