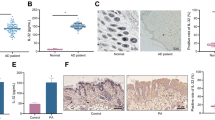
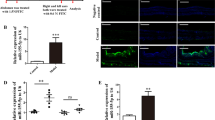
Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease influencing not only children but also adults. It is well-known that AD has a complex pathogenesis without effective therapy. Herein, we explored the function and mechanism of CYT387, a novel JAK2 inhibitor, on epidermal barrier damage. HaCaT cells exposed with high-concentration Ca2+ (1.8 mM) for 14 days were recruited for the model of keratinocytes (KC). The cell model of skin barrier damage was induced by IL-13, and KC markers such as filaggrin (FLG), loricrin (LOR), and involucrin (IVL) were detected to judge the success of the model. In this study, we found that miR-143 was lowly expressed whereas IL-13Rα1 was highly expressed in blood cells of patients with AD, indicating their negative correlation. Moreover, IL-13 treatment down-regulated miR-143 and up-regulated activated JAK2 and STAT3 phosphorylation, which was reversed by CYT387 administration. The dual-luciferase reporter assay verified that miR-143 could directly bind to 3′-UTR of IL-13Rα1, as well as STAT3. Furthermore, the function of CYT387 in the skin barrier damage induced by IL-13 was abolished by miR-143 inhibitor. Thus, CYT387 might alleviate IL-13-induced epidermal barrier damage via targeting IL-13Rα1 and STAT3 by miR-143 to repress inflammation. These findings revealed that the protective effects and the underlying mechanisms of CYT387 in AD, which provided evidence that miR-143 may be a novel therapeutic target for AD.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Amano W et al (2015) The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J Allergy Clin Immunol 136(3):667–677.e7
Bao L, Zhang H, Chan LS (2013) The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT 2(3):e24137
Bao L et al (2016) Interleukin-4 downregulation of involucrin expression in human epidermal keratinocytes involves Stat6 sequestration of the coactivator CREB-binding protein. J Interferon Cytokine Res 36(6):374–381
Bao L et al (2017) A molecular mechanism for IL-4 suppression of loricrin transcription in epidermal keratinocytes: implication for atopic dermatitis pathogenesis. Innate Immun 23(8):641–647
Batista DI et al (2015) Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol 29(6):1091–1095
Colombo I et al (2017) HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat Inflamm 2017:7435621
Dang NN et al (2015) Filaggrin silencing by shRNA directly impairs the skin barrier function of normal human epidermal keratinocytes and then induces an immune response. Braz J Med Biol Res 48(1):39–45
Goenka S, Kaplan MH (2011) Transcriptional regulation by STAT6. Immunol Res 50(1):87–96
Kabashima K (2012) Pathomechanism of atopic dermatitis in the perspective of T cell subsets and skin barrier functions—“Which comes first, the chicken or the egg?”. Dermatol Sin 30(4):142–146
Kent OA et al (2014) Lessons from miR-143/145: the importance of cell-type localization of miRNAs. Nucleic Acids Res 42(12):7528–7538
Kim BE et al (2008) Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 126(3):332–337
Lee E et al (2016) Atopic dermatitis phenotype with early onset and high serum IL-13 is linked to the new development of bronchial hyperresponsiveness in school children. Allergy 71(5):692–700
Liu Y et al (2017) Trim32 deficiency enhances Th2 immunity and predisposes to features of atopic dermatitis. J Investig Dermatol 137(2):359–366
Moreno AS et al (2016) Targeting the T helper 2 inflammatory axis in atopic dermatitis. Int Arch Allergy Immunol 171(2):71–80
Noh JY et al (2016) Thymic stromal lymphopoietin regulates eosinophil migration via phosphorylation of l-plastin in atopic dermatitis. Exp Dermatol 25(11):880–886
Pardanani A et al (2009) CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 23(8):1441–1445
Proksch E, Brandner JM, Jensen JM (2008) The skin: an indispensable barrier. Exp Dermatol 17(12):1063–1072
Rozalski M, Rudnicka L, Samochocki Z (2016) MiRNA in atopic dermatitis. Postepy Dermatol Alergol 33(3):157–162
Ruksha TG, Komina AV, Palkina NV (2017) MicroRNA in skin diseases. Eur J Dermatol 27(4):343–352
Skabytska Y et al (2016) How the innate immune system trains immunity: lessons from studying atopic dermatitis and cutaneous bacteria. J Dtsch Dermatol Ges 14(2):153–156
Strid J, McLean WH, Irvine AD (2016) Too much, too little or just enough: a goldilocks effect for IL-13 and skin barrier regulation? J Investig Dermatol 136(3):561–564
Sun L et al (2018) TSLP-activated dendritic cells induce T helper type 2 inflammation in Aspergillus fumigatus keratitis. Exp Eye Res 171:120–130
Teng Y et al (2015) miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Ralpha1. Biochem Biophys Res Commun 457(1):58–64
Thomsen SF et al (2016) Filaggrin gene loss-of-function mutations explain discordance of atopic dermatitis within dizygotic twin pairs. Int J Dermatol 55(12):1341–1344
Tindemans I, Peeters M, Hendriks RW (2017) Notch signaling in T helper cell subsets: instructor or unbiased amplifier? Front Immunol 8:419
Tyner JW et al (2010) CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 115(25):5232–5240
Verstraete K et al (2017) Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun 8:14937
Yu SQ et al (2011) Microarray analysis of differentially expressed microRNAs in allergic rhinitis. Am J Rhinol Allergy 25(6):e242–e246
Yu S et al (2013) MicroRNA-143 downregulates interleukin-13 receptor alpha1 in human mast cells. Int J Mol Sci 14(8):16958–16969
Zeng YP, Nguyen GH, Jin HZ (2016) MicroRNA-143 inhibits IL-13-induced dysregulation of the epidermal barrier-related proteins in skin keratinocytes via targeting to IL-13Ralpha1. Mol Cell Biochem 416(1–2):63–70
Funding
This work was supported by Excellent talent project of Organization Department of Beijing Municipal Party committee and Outstanding youth talents project of North University of Technology.
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: YZ; study concepts: YZ; study design: YZ; definition of intellectual content: XFC, QL; literature research: S-TZ; clinical studies: X-FC, S-TZ; experimental studies: YZ, W-TL; data acquisition: YZ, X-FC; data analysis: YZ, S-TZ; statistical analysis: YZ, S-TZ; manuscript preparation: YZ, W-TL; manuscript editing: YZ; manuscript review: YZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical Approval
The use of all samples in this article was approved by the Ethics Committee of Peking Union Medical College Hospital.
Consent for Publication
The informed consent obtained from study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10528_2020_10003_MOESM1_ESM.tif
Supplementary Fig. 1 A. Western blot analysis of pSTAT6 and STAT6, β-actin was used as internal control. After construction of the KC model, IL-13 (10 ng/mL) was administrated for inducing skin barrier damage, and cells were collected to analyze by western blot assay. B. qRT-PCR assay was performed to detect the expression of IL-13Rα2 and IL-4Rα after transfection of miR-143 mimics or inhibitor and their negative controls. N.S., no significance. (TIF 4116 kb)
Rights and permissions
About this article
Cite this article
Zu, Y., Chen, XF., Li, Q. et al. CYT387, a Novel JAK2 Inhibitor, Suppresses IL-13-Induced Epidermal Barrier Dysfunction Via miR-143 Targeting IL-13Rα1 and STAT3. Biochem Genet 59, 531–546 (2021). https://doi.org/10.1007/s10528-020-10003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-020-10003-0