Abstract
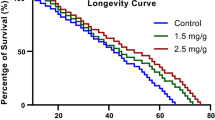
Piperine, a dietary phytochemical isolated from the Piper species, has been used as a natural medicine for pain, flu, and fever in ancient China and India. Although the health benefits of piperine have been widely studied, research on its effect on aging is limited. This study aimed to determine whether piperine has the potential to mitigate aging-related changes in the fruit fly (Drosophila melanogaster), which is an excellent model organism for studies on aging. The experiments were conducted using the newly eclosed or 30-day-old D. melanogaster wild-type strain Cantonized-white. Piperine was dissolved in 99% ethanol and added to the sucrose-yeast medium at a final concentration of 10, 35, 70, or 100 μM. The study examined the effects of piperine supplementation on the lifespan of D. melanogaster and other physiological functions, such as fecundity, feeding, lipid content, and resistance to environmental stress. Log-rank tests, Shapiro–Wilk test, F-test, t-test, or Wilcoxon rank sum test were used to analyze the data. Piperine failed to change the lifespan and body weight, but increased the fecundity and decreased the feeding rate in one-week-old flies. However, when piperine was fed to 30-day-old flies, it increased the lifespan of male flies and the fecundity and feeding rate of female flies. These results indicate that piperine can improve the health of aged flies. The findings suggest that piperine has age-dependent and sex-specific anti-aging effects in fruit flies.





Similar content being viewed by others
References
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Venter JC (2000) The genome sequence of Drosophila melanogaster. Science 287(5461):2185–2195. https://doi.org/10.1126/science.287.5461.2185
Atal S, Agrawal RP, Vyas S, Phadnis P, Rai N (2012) Evaluation of the effect of piperine per se on blood glucose level in alloxan-induced diabetic mice. Acta Pol Pharm 69(5):965–969
Baldal EA, Brakefield PM, Zwaan BJ (2006) Multitrait evolution in lines of Drosophila melanogaster selected for increased starvation resistance: the role of metabolic rate and implications for the evolution of longevity. Evolution 60(7):1435–1444
Banez MJ, Geluz MI, Chandra A, Hamdan T, Biswas OS, Bryan NS, Von Schwarz ER (2020) A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr Res 78:11–26. https://doi.org/10.1016/j.nutres.2020.03.002
Barbieri M, Bonafe M, Franceschi C, Paolisso G (2003) Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab 285(5):E1064-1071. https://doi.org/10.1152/ajpendo.00296.2003
Buranrat B, Junking M (2022) Piperine suppresses growth and migration of human breast cancer cells through attenuation of Rac1 expression. Asian Pac J Trop Med 12:39–46
Calabrese EJ, Baldwin LA (2002) Defining hormesis. Hum Exp Toxicol 21(2):91–97. https://doi.org/10.1191/0960327102ht217oa
Calabrese EJ, Mattson MP, Calabrese V (2010) Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum Exp Toxicol 29(12):980–1015. https://doi.org/10.1177/0960327110383625
Choi S, Choi Y, Choi Y, Kim S, Jang J, Park T (2013) Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem 141(4):3627–3635. https://doi.org/10.1016/j.foodchem.2013.06.028
Derosa G, Maffioli P, Sahebkar A (2016) Piperine and its role in chronic diseases. Adv Exp Med Biol 928:173–184. https://doi.org/10.1007/978-3-319-41334-1_8
Dhuley JN, Raman PH, Mujumdar AM, Naik SR (1993) Inhibition of lipid peroxidation by piperine during experimental inflammation in rats. Indian J Exp Biol 31(5):443–445
Du M, Liu X, Li C, Long S, Luo L, Guo Y, Wang D (2023) Uncovering the mechanisms of how capsaicin affects short-chain fatty acid production during food waste valorization. ACS ES&T Eng 3(11):1986–1996. https://doi.org/10.1021/acsestengg.3c00291
Duxbury EML, Chapman T (2020) Sex-specific responses of life span and fitness to variation in developmental versus adult diets in Drosophila melanogaster. J Gerontol A Biol Sci Med Sci 75(8):1431–1438. https://doi.org/10.1093/gerona/glz175
Elkhedir A, Iqbal A, Albahi A, Tao M, Rong L, Xu X (2022a) Capsaicinoid-glucosides of fresh hot pepper promotes stress resistance and longevity in Caenorhabditis elegans. Plant Foods Hum Nutr 77(1):30–36. https://doi.org/10.1007/s11130-021-00939-y
Elkhedir AE, Iqbal A, Zogona D, Mohammed HH, Murtaza A, Xu X (2022b) Apigenin glycosides from green pepper enhance longevity and stress resistance in Caenorhabditis elegans. Nutr Res 102:23–34. https://doi.org/10.1016/j.nutres.2022.02.003
Felix TM, Hughes KA, Stone EA, Drnevich JM, Leips J (2012) Age-specific variation in immune response in Drosophila melanogaster has a genetic basis. Genetics 191(3):989–1002. https://doi.org/10.1534/genetics.112.140640
Goncalves AC, Nunes AR, Falcao A, Alves G, Silva LR (2021) Dietary effects of anthocyanins in human health: a comprehensive review. Pharmaceuticals (basel). https://doi.org/10.3390/ph14070690
Gundala SR, Aneja R (2014) Piper betel leaf: a reservoir of potential xenohormetic nutraceuticals with cancer-fighting properties. Cancer Prev Res (phila) 7(5):477–486. https://doi.org/10.1158/1940-6207.CAPR-13-0355
Haq IU, Imran M, Nadeem M, Tufail T, Gondal TA, Mubarak MS (2021) Piperine: a review of its biological effects. Phytother Res 35(2):680–700. https://doi.org/10.1002/ptr.6855
Harshman LG, Hoffmann AA, Clark AG (1999) Selection for starvation resistance in Drosophila melanogaster: physiological correlates, enzyme activities and multiple stress responses. J Evol Biol 12(2):370–379
He Q, Xu JY, Gu J, Tong X, Wan ZX, Gu Y, Fang C, Qin LQ (2022) Piperine is capable of improving pancreatic beta-cell apoptosis in high fat diet and streptozotocin induced diabetic mice. J Funct Foods. https://doi.org/10.1016/j.jff.2021.104890
Hewlings SJ, Kalman DS (2017) Curcumin: a review of its effects on human health. Foods. https://doi.org/10.3390/foods6100092
Hoffman JM, Soltow QA, Li S, Sidik A, Jones DP, Promislow DE (2014) Effects of age, sex, and genotype on high-sensitivity metabolomic profiles in the fruit fly Drosophila melanogaster. Aging Cell 13(4):596–604. https://doi.org/10.1111/acel.12215
Jwa H, Choi Y, Park UH, Um SJ, Yoon SK, Park T (2012) Piperine, an LXRalpha antagonist, protects against hepatic steatosis and improves insulin signaling in mice fed a high-fat diet. Biochem Pharmacol 84(11):1501–1510. https://doi.org/10.1016/j.bcp.2012.09.009
Kristensen TN, Loeschcke V, Tan Q, Pertoldi C, Mengel-From J (2019) Sex and age specific reduction in stress resistance and mitochondrial DNA copy number in Drosophila melanogaster. Sci Rep 9(1):12305. https://doi.org/10.1038/s41598-019-48752-7
Krzyzanowska J, Czubacka A, Oleszek W (2010) Dietary phytochemicals and human health. Adv Exp Med Biol 698:74–98. https://doi.org/10.1007/978-1-4419-7347-4_7
Lee KS, Lee BS, Semnani S, Avanesian A, Um CY, Jeon HJ, Seong KM, Yu K, Min KJ, Jafari M (2010) Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res 13(5):561–570. https://doi.org/10.1089/rej.2010.1031
Lee HY, Lee JH, Kim SH, Jo SY, Min KJ (2023) Probiotic Limosilactobacillus reuteri (Lactobacillus reuteri) extends the lifespan of Drosophila melanogaster through Insulin/IGF-1 signaling. Aging Dis. https://doi.org/10.14336/AD.2023.0122
Leech T, Evison SEF, Armitage SAO, Sait SM, Bretman A (2019) Interactive effects of social environment, age and sex on immune responses in Drosophila melanogaster. J Evol Biol 32(10):1082–1092. https://doi.org/10.1111/jeb.13509
Leech T, McDowall L, Hopkins KP, Sait SM, Harrison XA, Bretman A (2021) Social environment drives sex and age-specific variation in Drosophila melanogaster microbiome composition and predicted function. Mol Ecol 30(22):5831–5843. https://doi.org/10.1111/mec.16149
Lehtovaara A, Schielzeth H, Flis I, Friberg U (2013) Heritability of life span is largely sex limited in Drosophila. Am Nat 182(5):653–665. https://doi.org/10.1086/673296
Leonov A, Arlia-Ciommo A, Piano A, Svistkova V, Lutchman V, Medkour Y, Titorenko VI (2015) Longevity extension by phytochemicals. Molecules 20(4):6544–6572. https://doi.org/10.3390/molecules20046544
Lizcano LJ, Siles M, Trepiana J, Hernandez ML, Navarro R, Ruiz-Larrea MB, Ruiz-Sanz JI (2014) Piper and Vismia species from Colombian Amazonia differentially affect cell proliferation of hepatocarcinoma cells. Nutrients 7(1):179–195. https://doi.org/10.3390/nu7010179
Lushchak O, Strilbytska O, Storey KB (2023) Gender-specific effects of pro-longevity interventions in Drosophila. Mech Ageing Dev 209:111754. https://doi.org/10.1016/j.mad.2022.111754
Ma Y, Lee G, Heo SY, Roh YS (2021) Oxidative stress is a key modulator in the development of nonalcoholic fatty liver disease. Antioxidants (basel). https://doi.org/10.3390/antiox11010091
Magwere T, Chapman T, Partridge L (2004) Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci 59(1):3–9. https://doi.org/10.1093/gerona/59.1.b3
Martel J, Ojcius DM, Ko YF, Ke PY, Wu CY, Peng HH, Young JD (2019) Hormetic effects of phytochemicals on health and longevity. Trends Endocrinol Metab 30(6):335–346. https://doi.org/10.1016/j.tem.2019.04.001
Millington JW, Rideout EJ (2018) Sex differences in Drosophila development and physiology. Curr Opin Physiol 6:46–56. https://doi.org/10.1016/j.cophys.2018.04.002
Mittal R, Gupta RL (2000) In vitro antioxidant activity of piperine. Methods Find Exp Clin Pharmacol 22(5):271–274. https://doi.org/10.1358/mf.2000.22.5.796644
Moghaddam NSA, Oskouie MN, Butler AE, Petit PX, Barreto GE, Sahebkar A (2019) Hormetic effects of curcumin: What is the evidence? J Cell Physiol 234(7):10060–10071. https://doi.org/10.1002/jcp.27880
Neckameyer WS, Nieto-Romero AR (2015) Response to stress in Drosophila is mediated by gender, age and stress paradigm. Stress Int J Biol Stress 18(2):254–266. https://doi.org/10.3109/10253890.2015.1017465
Panfoli I, Puddu A, Bertola N, Ravera S, Maggi D (2021) The hormetic effect of metformin: “Less Is More”? Int J Mol Sci. https://doi.org/10.3390/ijms22126297
Park HM, Kim JH, Kim DK (2019) Anti-oxidative effect of piperine from Piper nigrum L. Caenorhabditis elegans. Nat Product Sci 25(3):255–260. https://doi.org/10.20307/nps.2019.25.3.255
Reiter LT, Potocki L, Chien S, Gribskov M, Bier E (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 11(6):1114–1125. https://doi.org/10.1101/gr.169101
Samuel M, Oliver SV, Coetzee M, Brooke BD (2016) The larvicidal effects of black pepper (Piper nigrum L.) and piperine against insecticide resistant and susceptible strains of Anopheles malaria vector mosquitoes. Parasit Vectors 9:238. https://doi.org/10.1186/s13071-016-1521-6
Sanchez-Roman I, Barja G (2013) Regulation of longevity and oxidative stress by nutritional interventions: role of methionine restriction. Exp Gerontol 48(10):1030–1042. https://doi.org/10.1016/j.exger.2013.02.021
Schulz M, Łoś A, Grzybek M, Ścibior R, Strachecka A (2019) Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J Agric Sci 157(2):140–149. https://doi.org/10.1017/S0021859619000431
Selvendiran K, Singh JP, Krishnan KB, Sakthisekaran D (2003) Cytoprotective effect of piperine against benzo[a]pyrene induced lung cancer with reference to lipid peroxidation and antioxidant system in Swiss albino mice. Fitoterapia 74(1–2):109–115. https://doi.org/10.1016/s0367-326x(02)00304-0
Semaniuk UV, Gospodaryov DV, Strilbytska OM, Kucharska AZ, Sokol-Letowska A, Burdyliuk NI, Storey KB, Bayliak MM, Lushchak O (2022) Chili pepper extends lifespan in a concentration-dependent manner and confers cold resistance on Drosophila melanogaster cohorts by influencing specific metabolic pathways. Food Funct 13(15):8313–8328. https://doi.org/10.1039/d2fo00930g
Shahrestani P, Quach J, Mueller LD, Rose MR (2012) Paradoxical physiological transitions from aging to late life in Drosophila. Rejuvenation Res 15(1):49–58. https://doi.org/10.1089/rej.2011.1201
Shen J, Shan J, Zhu X, Yang P, Zhang D, Liang B, Li M, Zang X, Dai Z (2020) Sex specific effects of capsaicin on longevity regulation. Exp Gerontol 130:110788. https://doi.org/10.1016/j.exger.2019.110788
Srinivasan K (2007) Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr 47(8):735–748. https://doi.org/10.1080/10408390601062054
Tavares WS, Cruz I, Petacci F, Freitas SS, Serrao JE, Zanuncio JC (2011) Insecticide activity of piperine: toxicity to eggs of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea saccharalis (Lepidoptera: Pyralidae) and phytotoxicity on several vegetables. J Med Plants Res 5(21):5301–5306
Tennessen JM, Barry WE, Cox J, Thummel CS (2014) Methods for studying metabolism in Drosophila. Methods 68(1):105–115. https://doi.org/10.1016/j.ymeth.2014.02.034
Veerkamp JH, Maatman RG (1995) Cytoplasmic fatty acid-binding proteins: their structure and genes. Prog Lipid Res 34(1):17–52. https://doi.org/10.1016/0163-7827(94)00005-7
Verma N, Bal S, Gupta R, Aggarwal N, Yadav A (2020) Antioxidative effects of piperine against cadmium-induced oxidative stress in cultured human peripheral blood lymphocytes. J Diet Suppl 17(1):41–52. https://doi.org/10.1080/19390211.2018.1481485
Wang X, Zhang Y, Zhang L, Wang W, Che H, Zhang Y (2022) Piperine attenuates hepatic steatosis and insulin resistance in high-fat diet-induced obesity in Sprague-Dawley rats. Nutr Res 108:9–21. https://doi.org/10.1016/j.nutres.2022.10.007
Yang W, Chen YH, Liu H, Qu HD (2015) Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int J Mol Med 36(5):1369–1376. https://doi.org/10.3892/ijmm.2015.2356
Zarai Z, Boujelbene E, Salem NB, Gargouri Y, Sayari A (2013) Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci Technol 50(2):634–641. https://doi.org/10.1016/j.lwt.2012.07.036
Zhang C, Tian Q, Li Y (2022) Design, synthesis, and insecticidal activity evaluation of piperine derivatives. Front Chem 10:973630. https://doi.org/10.3389/fchem.2022.973630
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022H1D8A3037396).
Author information
Authors and Affiliations
Contributions
Lee. H.Y, Lee. J. H. Cho. K.A. and Min. K.J . wrote the main manuscript text and Lee. H.Y, Lee. J. H, Baek. J.S. prepared figures 1-5. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, HY., Lee, JH., Baek, J. et al. Piperine improves the health span of Drosophila melanogaster with age- and sex-specific effect. Biogerontology 25, 665–677 (2024). https://doi.org/10.1007/s10522-024-10100-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-024-10100-2