Abstract
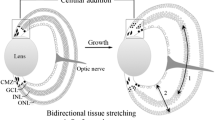
The fast-ageing killifish has gained increasing attention as a promising gerontology model to study age-related processes and neurodegeneration. Interestingly, it is the first vertebrate model organism that shows physiological neuron loss at old age in its central nervous system (CNS), including its brain and retina. However, the fact that the killifish brain and retina are ever-growing tissues complicates studying neurodegenerative events in aged fish. Indeed, recent studies showed that the method of tissue sampling, either using sections or whole-organs, has a large effect on the observed cell densities in the fast-expanding CNS. Here, we elaborated on how these two sampling methods affect neuronal counts in the senescent retina and how this tissue grows upon ageing. Analysis of the different retinal layers in cryosections revealed age-dependent reduction in cellular density but evaluation of whole-mount retinas did not detect any neuron loss, as a result of an extremely fast retinal expansion with age. Using BrdU pulse-chase experiments, we showed that the young adult killifish retina mainly grows by cell addition. However, with increasing age, the neurogenic potency of the retina declines while the tissue keeps on growing. Further histological analyses revealed tissue stretching, including cell size increase, as the main driver of retinal growth at old age. Indeed, both cell size and inter-neuronal distance augment with ageing, thereby decreasing neuronal density. All in all, our findings urge the ‘ageing science’ community to consider cell quantification bias and employ tissue-wide counting methods to reliably quantify neuronal numbers in this unique gerontology model.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Armstrong HA, Smith CJ (2001) Growth patterns in euconodont crown enamel: implications for life history and mode-of-life reconstruction in the earliest vertebrates. Proc R Soc B Biol Sci 268:815–820. https://doi.org/10.1098/rspb.2001.1591
Bagnoli S, Fronte B, Bibbiani C et al (2022) Quantification of noradrenergic-, dopaminergic-, and tectal-neurons during aging in the short-lived killifish Nothobranchius furzeri. Aging Cell 21:e13689. https://doi.org/10.1111/ACEL.13689
Cellerino A, Valenzano DR, Reichard M (2016) From the bush to the bench: the annual Nothobranchius fishes as a new model system in biology. Biol Rev 91:511–533. https://doi.org/10.1111/brv.12183
Dobbing J, Sands J (1985) Cell size and cell number in tissue growth and development. an old hypothesis reconsidered. Arch Fr Pediatr 42:199–203
Fernández-Nogales M, Murcia-Belmonte V, Chen HY, Herrera E (2019) The peripheral eye: a neurogenic area with potential to treat retinal pathologies? Prog Retin Eye Res 68:110–123. https://doi.org/10.1016/j.preteyeres.2018.09.001
Genade T, Benedetti M, Terzibasi E et al (2005) Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell 4:223–233. https://doi.org/10.1111/j.1474-9726.2005.00165.x
Johns PR (1977) Growth of the adult goldfish eye. III. Source of the new retinal cells. J Comp Neurol 176:343–357. https://doi.org/10.1002/cne.901760304
Johns PR (1981) Growth of fish retinas. Am Zool 21:447–458
Kim Y, Nam HG, Valenzano DR (2016) The short-lived African turquoise killifish: an emerging experimental model for ageing. DMM Dis Model Mech 9:115–129. https://doi.org/10.1242/dmm.023226
Kock J-H, Reuter T (1978) Retinal ganglion cells in the crucian carp (Carassius carassius). I. Size and number of somata in eyes of different size. J Comp Neurol 179:535–547. https://doi.org/10.1002/cne.901790306
Lenkowski JR, Raymond PA (2014) Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res 40:94–123
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194. https://doi.org/10.1016/j.cell.2013.05.039
Lyall AH (1957) The growth of the trout reina. J Cell Sci 98:101–110. https://doi.org/10.1242/JCS.S3-98.41.101
Marcucci F, Murcia-Belmonte V, Wang Q et al (2016) The ciliary margin zone of the mammalian retina generates retinal ganglion cells. Cell Rep 17:3153–3164. https://doi.org/10.1016/j.celrep.2016.11.016
Mariën V, Van houcke J, Arckens L (2022) Intracardial perfusion of the African turquoise killifish. https://www.protocols.io/view/intracardial-perfusion-of-the-african-turquoise-ki-b2ryqd7w. Accessed 20 Jan 2022
Masin L, Claes M, Bergmans S et al (2021) A novel retinal ganglion cell quantification tool based on deep learning. Sci Rep 11:702. https://doi.org/10.1038/s41598-020-80308-y
Matsui H, Kenmochi N, Namikawa K (2019) Age- and α-synuclein-dependent degeneration of dopamine and noradrenaline neurons in the annual killifish nothobranchius furzeri. Cell Rep 26:1727–1733. https://doi.org/10.1016/j.celrep.2019.01.015
Müller H (1952) Bau und Wachstum der netzhaut des Guppy (Lebistes reticulatus). Zool Jarhbücher 63:275–324
Platzer M, Englert C (2016) Nothobranchius furzeri: a model for aging research and more. Trends Genet 32:543–552. https://doi.org/10.1016/j.tig.2016.06.006
Ronneberger O, Fischer P, Brox T (2015) U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv. https://doi.org/10.48550/arXiv.1505.04597
Sands J, Dobbing J, Gratrix CA (1979) Cell number and cell size: organ growth and development and the ctonral of catch-up growth in rats. Lancet 314:503–505. https://doi.org/10.1016/S0140-6736(79)91556-3
Stenkamp DL (2007) Neurogenesis in the fish retina. Int Rev Cytol 259:173–224. https://doi.org/10.1016/S0074-7696(06)59005-9
Stenkamp DL (2011) The rod photoreceptor lineage of teleost fish. Prog Retin Eye Res 30:395–404. https://doi.org/10.1016/J.PRETEYERES.2011.06.004
Terzibasi E, Valenzano DR, Benedetti M et al (2008) Large differences in aging phenotype between strains of the short-lived annual fish Nothobranchius furzeri. PLoS ONE 3:e3866. https://doi.org/10.1371/journal.pone.0003866
Valenzano DR, Terzibasi E, Genade T et al (2006) Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 16:296–300. https://doi.org/10.1016/j.cub.2005.12.038
Van Houcke J, Geeraerts E, Vanhunsel S et al (2019) Extensive growth is followed by neurodegenerative pathology in the continuously expanding adult zebrafish retina. Biogerontology 20:109–125. https://doi.org/10.1007/S10522-018-9780-6/FIGURES/10
Van Houcke J, Mariën V, Zandecki C et al (2021) Aging impairs the essential contributions of non-glial progenitors to neurorepair in the dorsal telencephalon of the Killifish Nothobranchius furzeri. Aging Cell 20:e13464. https://doi.org/10.1111/ACEL.13464
Vanhunsel S, Bergmans S, Beckers A et al (2021) The killifish visual system as an in vivo model to study brain aging and rejuvenation. NPJ Aging Mech Dis 7:1–17. https://doi.org/10.1038/s41514-021-00077-4
Vanhunsel S, Bergmans S, Beckers A et al (2022) The age factor in optic nerve regeneration: Intrinsic and extrinsic barriers hinder successful recovery in the short-living killifish. Aging Cell 21:e13537. https://doi.org/10.1111/ACEL.13537
Vrtílek M, Žák J, Pšenička M, Reichard M (2018) Extremely rapid maturation of a wild African annual fish. Curr Biol 28:R822–R824. https://doi.org/10.1016/J.CUB.2018.06.031
Wan Y, Almeida AD, Rulands S et al (2016) The ciliary marginal zone of the zebrafish retina: Clonal and time-lapse analysis of a continuously growing tissue. Dev 143:1099–1107. https://doi.org/10.1242/dev.133314
Acknowledgements
The authors would like to thank Simon Buys, Arnold Van Den Eynde and Rony Van Aerschot for the daily fish maintenance and environmental control; Stephanie Mentens, Marijke Christiaens and Nele Michiels for their technical support. S.B. and Lu.M hold a personal Research Foundation Flanders (FWO, Belgium) fellowship (1165020N and 1S42720N, respectively). Killifish housing and breeding is financially supported by a small equipment Grant KA-16-00745 (KU Leuven) while experiments are supported by a FWO research grant (G092222N).
Author information
Authors and Affiliations
Contributions
Conceptualization: SB, LuM and LM; Methodology: SB, LuM, P-JS; Writing—original draft preparation: SB; Writing—review and editing: SB, LuM, P-JS, LM; Funding acquisitions: Research Foundation Flanders, KU Leuven; Visualization: SB, LuM; and Supervision: LM.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no actual or potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bergmans, S., Serneels, PJ., Masin, L. et al. Tissue stretching is a confounding factor for the evaluation of neurodegeneration in the fast-ageing killifish. Biogerontology 24, 403–419 (2023). https://doi.org/10.1007/s10522-023-10026-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-023-10026-1