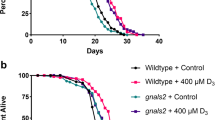
Abstract
Age predisposes individuals to significant diseases, and the biological processes contributing to aging are currently under intense investigation. Klotho is an anti-aging protein with multifaceted roles and is an essential component of the endocrine fibroblast growth factor. In Caenorhabditis elegans (C. elegans), there are two prospective orthologs of α-Klotho, C50F7.10, and E02H9.5, identified. The two orthologs' products are homologous to the highly conserved KL1 domain of human and mouse Klotho protein. Considering the endocrine system's major involvement in an organism's homeostasis and that thyroid disorders increase with advancing age, the molecular mechanisms underlying its impact on different endocrine components during the aging process remain poorly characterized. In this study, we sought to determine the regulatory role of Triiodothyronine (T3) on homologs genes of klotho and its impact on different parameters of aging in the C. elegans model organism. We showed that T3 could increase the mRNA expressions of the klotho homologous genes in C. elegans. Moreover, T3 could also extend a worm lifespan and modulate oxidative stress resistance and aging biomarkers significantly and positively. Further investigations employing different mutant and transgenic strains reveal that these observed effects are mediated through the EGL-17/EGL-15 pathway via Klotho activation along with the involvement of transcription factor DAF-16. In conclusion, these findings have revealed an unexpected link between T3 and Klotho and how this link can modulate the aging process in C. elegans via activation of klotho. This study will help understand the crosstalk and regulations of different endocrine components and their consequences on the aging process in multiple species.









Similar content being viewed by others
Abbreviations
- T3:
-
Triiodothyronine
- tBOOH:
-
Tert-Butyl hydroperoxide
- FGFR:
-
Fibroblast growth factor receptor
- µM:
-
Micro molar
- mM:
-
Milli molar
- SEM:
-
Standard error of mean
- FUDR:
-
5-Fluoro-2′-deoxyuridine
- Hours:
-
Hrs
References
Adijiang A, Niwa T (2010) An oral sorbent, AST-120, increases klotho expression and inhibits cell senescence in the kidney of uremic rats. Am J Nephrol 31:160–164
Altman DG, Bland JM (1998) Statistics notes: generalisation and extrapolation. BMJ 317:409–410
An JH, Blackwell TK (2003) SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev 17:1882–1893
Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ (2008) Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell 7:13–22
Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS (2015) The emonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30:223–233
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C (2009) Klotho is a substrate for α-, β- and γ-secretase. FEBS Lett 583:3221–3224
Blüher M, Kahn BB, Kahn CR (2003) Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299:572–574
Bolanowski MA, Russell RL, Jacobson LA (1981) Quantitative measures of aging in the nematode Caenorhabditis elegans. In population and longitudinal studies of two behavioral parameters. Mech Ageing Dev 15:279–295
Bowers J, Terrien J, Clerget-Froidevaux MS, Gothie JD, Rozing MP, Westendorp RGJ, Van Heemst D, Demeneix BA (2013) Thyroid hormone signaling and homeostasis during aging. Endocr Rev 34:556–589
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:77–94
Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME (2001) Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965
Burdine RD, Chen EB, Kwok SF, Stern MJ (1997) Egl-17 encodes an invertebrate fibroblast growth factor family member required specifically for sex myoblast migration in Caenorhabditis elegans. Proc Natl Acad Sci USA 94:2433–2437
Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13:1763–1811
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang CL (2008) Removal of sialic acid involving klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105:9805–9810
Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL (2009) Regulation of renal outer medullary potassium channel and renal K(+) excretion by klotho. Mol Pharmacol 76:38–46
Chaker L, Cappola AR, Mooijaart SP, Peeters RP (2018) Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol 6:733–742
Chang Q (2005) The beta-Glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science 310:490–493
Château MT, Araiz C, Descamps S, Galas S (2010) Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging (Albany NY) 2:567–581
Chayen J (1995) Cell biology: A laboratory handbook. J. E. Celis (Ed.). Academic Press: New York, London, Sydney, Tokyo, Toronto. Cell Biochem Funct 13:299–299
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR (2007) Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci 104:19796–19801
Chen ATY, Guo C, Dumas KJ, Ashrafi K, Hu PJ (2013) Effects of Caenorhabditis elegans sgk-1 mutations on lifespan, stress resistance, and DAF-16/FoxO regulation. Aging Cell 12:932–940
Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, Abraham CR (2014) Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 53:5579–5587
Chow DK, Glenn CF, Johnston JL, Goldberg IG, Wolkow CA (2006) Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Exp Gerontol 41:252–260
Clokey GV, Jacobson LA (1986) The autofluorescent lipofuscin granules in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev 35:79–94
Croll NA, Smith JM, Zuckerman BM (1977) The aging process of the nematode Caenorhabditis elegans in bacterial and axenic culture. Exp Aging Res 3:175–189
Dërmaku-Sopjani M, Sopjani M, Saxena A, Shojaiefard M, Bogatikov E, Alesutan I, Eichenmüller M, Lang F (2011) Downregulation of NaPi-IIa and NaPi-IIb Na-coupled phosphate transporters by coexpression of Klotho. Cell Physiol Biochem 28:251–258
Duangjan C, Rangsinth P, Gu X, Wink M, Tencomnao T (2019) Lifespan extending and oxidative stress resistance properties of a leaf extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxid Med Cell Longev. https://doi.org/10.1155/2019/9012396
Essers MAG, Weijzen S, De Vries-Smits AMM, Saarloos I, De Ruiter ND, Bos JL, Burgering BMT (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812
Essers MAG, De Vries-Smits LMM, Barker N, Polderman PE, Burgering BMT, Korswagen HC (2005) Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science 308:1181–1184
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span-from yeast to humans. Science 328:321–326
Gavrilov LA, Gavrilova NS (2001) The reliability theory of aging and longevity. J Theor Biol 213:527–545
Gesing A (2015) The thyroid gland and the process of aging. Thyroid Res 8:A8
Golden TR, Hubbard A, Dando C, Herren MA, Melov S (2008) Age-related behaviors have distinct transcriptional profiles in Caenorhabditis elegans. Aging Cell 7:850–865
Gray DA (2005) Woulfe J (2005) Lipofuscin and aging: a matter of toxic waste. Sci Aging Knowl Environ. 5:re1
Gruber J, Schaffer S, Halliwell B (2008) The mitochondrial free radical theory of ageing–where do we stand? Front Biosci 13:6554–6579
Guarente L, Kenyon C (2000) Genetic pathways that regulate ageing in model organisms. Nature 408:255–262
Hahm JH, Kim S, Diloreto R, Shi C, Lee SJV, Murphy CT, Nam HG (2015) C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat Commun 6:8919
Hayes JD, McMahon M (2001) Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett 174:103–113
Henderson ST, Johnson TE (2001) daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol 11:1975–1980
Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421:182–187
Hosono R, Sato Y, Aizawa SI, Mitsui Y (1980) Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol 15:285–289
Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW (2010a) Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78:1240–1251
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW (2010b) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24:3438–3450
Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22:124–136
Huang C, Xiong C, Kornfeld K (2004) Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci USA 101:8084–8089
Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T (2006) Anti-apoptotic and anti-senescence effects of klotho on vascular endothelial cells. Biochem Biophys Res Commun 339:827–832
Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima YI (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of klotho protein from cell membrane. FEBS Lett 565:143–147
Kahn NW, Rea SL, Moyle S, Kell A, Johnson TE (2008) Proteasomal dysfunction activates the transcription factor SKN-1 and produces a selective oxidative-stress response in Caenorhabditis elegans. Biochem J 409:205–213
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Keith SA, Amrit FRG, Ratnappan R, Ghazi A (2014) The C. elegans healthspan and stress-resistance assay toolkit. Methods 68:476–486
Kenyon C (2005) The plasticity of aging: Insights from long-lived mutants. Cell 120:449–460
Kenyon C (2011) The first long-lived mutants: Discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R Soc B Biol Sci 366:9–16
Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G (1997) Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277:942–946
King GD, Chen CD, Huang MM, Zeldich E, Brazee PL, Schuman ER, Robin M, Cuny GD, Glicksman MA, Abraham CR (2012) Identification of novel small molecules that elevate Klotho expression. Biochem J 441:453–461
Klass MR (1977) Aging in the nematode Caenorhabditis elegans: Major biological and environmental factors influencing life span. Mech Ageing Dev 6:413–429
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kurosu H (2005) Suppression of aging in mice by the hormone klotho. Science 309:1829–1833
Maltese G, Psefteli PM, Rizzo B, Srivastava S, Gnudi L, Mann GE, Siow RCM (2017) The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med 21:621–627
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y (1998) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242:626–630
Mukhopadhyay A, Oh SW, Tissenbaum HA (2006) Worming pathways to and from DAF-16/FOXO. Exp Gerontol 41:928–934
Nabeshima Y (2008) The discovery of α-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis. Cell Mol Life Sci 65:3218–3230
Nabeshima Y, Imura H (2008) Alpha-klotho: a regulator that integrates calcium homeostasis. Am J Nephrol 28:455–464
Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R (1998) Molecular cloning of rat klotho cDNA markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun 251:920–925
Oliveira RP, Abate JP, Dilks K, Landis J, Ashraf J, Murphy CT, Blackwell TK (2009) Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 8:524–541
Papaevgeniou N, Hoehn A, Grune T, Chondrogianni N (2017) Lipofuscin effects in Caenorhabditis elegans ageing model. Free Radic Biol Med 108:S48
Park JH, Lee JH, Park JW, Kim DY, Hahm JH, Nam HG, Bae YS (2017) Downregulation of protein kinase CK2 activity induces agerelated biomarkers in C. elegans. Oncotarget 8:36950–36963
Peixoto H, Roxo M, Krstin S, Röhrig T, Richling E, Wink M (2016) An anthocyanin-rich extract of acai (Euterpe precatoria Mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. J Agric Food Chem 64:1283–1290
Rangsinth P, Prasansuklab A, Duangjan C, Gu X, Meemon K, Wink M, Tencomnao T (2019) Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement Altern Med 19:164
Rattan SIS (2012) Rationale and methods of discovering hormetins as drugs for healthy ageing. Expert Opin Drug Discov 7:439–448
Roubin R, Naert K, Popovici C, Vatcher G, Coulier F, Thierry-Mieg J, Pontarotti P, Birnbaum D, Baillie D, Thierry-Mieg D (1999) let-756, a C. elegans fgf essential for worm development. Oncogene 18:6741–6747
Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K (2007) Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Physiol 292:F769–F779
Semba RD, Moghekar AR, Hu J, Sun K, Turner R, Ferrucci L, O’Brien R (2014) Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci Lett 558:37–40
Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-O M, Nabeshima YI (1998) Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424:6–10
Silverman GA, Luke CJ, Bhatia SR, Long OS, Vetica AC, Perlmutter DH, Pak SC (2009) Modeling molecular and cellular aspects of human disease using the nematode Caenorhabditis elegans. Pediatr Res 65:10–18
Sohal RS, Brunk UT (1989) Lipofuscin as an indicator of oxidative stress and aging. Adv Exp Med Biol 266:17–26
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273:59–63
Sundelin SP, Nilsson SEG (2001) Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Radic Biol Med 31:217–225
Szewczyk NJ, Peterson BK, Barmada SJ, Parkinson LP, Jacobson LA (2007) Opposed growth factor signals control protein degradation in muscles of Caenorhabditis elegans. EMBO J 26:935–943
Takahashi Y, Kuro-O M, Ishikawa F (2000) Aging mechanisms. Proc Natl Acad Sci USA 97:12407–12408
Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS (2001) A mutant Drosophila insulin receptor homolog that extends lifespan and impairs neuroendocrine function. Science 292:107–110
Tissenbaum HA (2015) Using C. elegans for aging research. Invertebr Reprod Dev 59(sup1):59–63
Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima YI (2003) Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of Vitamin D endocrine system. Mol Endocrinol 17:2393–2403
Viña J, Borrás C, Miquel J (2007) Theories of ageing. IUBMB Life 59:249–254
Wickens AP (2001) Ageing and the free radical theory. Respir Physiol 128:379–391
Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-O M, Karlan B, Kaufman B, Koeffler HP, Rubinek T (2008) Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene 27:7094–7105
Xu H, Chen M, Manivannan A, Lois N, Forrester JV (2008) Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell 7:58–68
Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-O M (2005) Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280:38029–38034
Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, Ohashi N, Kobori H, Kuro-O M, Yang CW (2011) Angiotensin II blockade upregulates the expression of klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant 26:800–813
Yoshida T, Fujimori T, Nabeshima YI (2002) Mediation of unusually high concentrations of 1,25-Dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1α-hydroxylase gene. Endocrinology 143:683–689
Acknowledgements
The authors acknowledge the Department of Biochemistry and Molecular Biology, Pondicherry University, India and Department of Science & Technology (DST), Science and Engineering Research Board (SERB), Government of India, for financial and overall support.
Funding
This work was supported by the Department of Biochemistry and Molecular Biology, Pondicherry University, India. KS is a recipient of funding from SERB (File No EEQ/2016/000343). SKM was supported by a fellowship from the Council of Scientific and Industrial Research (CSIR) (File No 09/559(0128)/2019-EMR-I), Ministry of Science and Technology, Government of India.
Author information
Authors and Affiliations
Contributions
SKM: Conceptualization, methodology, investigation, formal analysis, writing—original draft, project administration. KS: Supervision, conceptualization, validation, analysis, review and editing, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohanty, S.K., Suchiang, K. Triiodothyronine (T3) enhances lifespan and protects against oxidative stress via activation of Klotho in Caenorhabditis elegans. Biogerontology 22, 397–413 (2021). https://doi.org/10.1007/s10522-021-09923-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-021-09923-0