Abstract
Breastfeeding is hypothesised to benefit child health and cognitive functioning by providing long-chain polyunsaturated fatty acids, which are essential for brain development. In 2007, Caspi et al. found evidence in two cohorts for an interaction between genetic variation in the FADS2 gene (a gene involved in fatty acid metabolism) and breastfeeding on IQ. However, subsequent studies have provided mixed evidence for the existence of an interaction. We investigated the relationship between genetic variation in the FADS2 region, breastfeeding, and their interaction in up to 335,650 individuals from the UK Biobank. We tested for the interaction over a range of cognitive functioning tests, as well as educational attainment and other traits thought to be influenced by breastfeeding, including cardiometabolic traits, number of offspring, and atopic allergy. FADS2 alleles associated with an increase in docosahexaenoic acid in blood serum (the C allele of rs174575) were associated with decreased verbal-numerical reasoning (\(p=2.28\times {10}^{-5}\)) and triglycerides (\(p=1.40\times {10}^{-41}\)), increased number of offspring (\(p=3.40\times {10}^{-5}\)), total cholesterol (\(p=5.28\times {10}^{-36}\)), HDL (\(p=1.42\times {10}^{-51}\)), and LDL cholesterol (\(p=1.46\times {10}^{-21}\)). We observed no evidence of an interaction in any of the traits, regardless of the modelling strategy on any cognitive or non-cognitive traits. We postulate that the previous positive findings are likely to be spurious, perhaps due to lack of appropriate control for latent population structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breastfeeding is the recommended method of feeding infants for the first six months of their life (World Health Organization 2001, 2003). Over the last century, the scientific literature has reported evidence for the benefits of breastfeeding on infant mortality and health (Anderson et al. 1982; Michaelsen et al. 2009; Stevens et al. 2009; Binns et al. 2016; Victora et al. 2016). An increasing body of evidence also suggests that breastfeeding may exert positive effects on cognitive outcomes, including observational studies that control for maternal intelligence and socioeconomic status (SES; Horta et al. 2015, 2018), and a large randomised cluster trial (Kramer et al. 2008).
One of the hypothesised mechanisms explaining the putative efficacy of breastfeeding on cognitive function, compared to other forms of infant feeding, is the presence of long-chain polyunsaturated fatty acids (LC-PUFA) in breastmilk. In particular, the presence of docosahexaenoic acid (DHA), an n-3 fatty acid (22:6(n-3)), and arachidonic acid (AA), an n-6 fatty acid (20:4(n-6); Koletzko et al. 2001; Lattka et al. 2012), both of which were traditionally absent from commercial infant feeding formulas (Lauritzen et al. 2001). DHA and AA are required for the development of the brain and retina (Lauritzen et al. 2001; Brenna and Diau 2007) to synthesise membrane phospholipids such as phosphoinositides, which regulate membrane proteins dedicated to transport and signalling (Falkenburger et al. 2010), and phosphatidylserine, which have a structural role in cell membrane (Kim et al. 2014; Sun et al. 2018). They can be sourced through direct dietary intake of preformed acids or synthesised from precursors (e.g., from the conversion of linoleic acid [LA] and eicosapentaenoic acid [EPA], respectively; Forsyth et al. 2016). It is generally understood that biosynthesis of DHA and AA in adult humans is limited (Burdge and Calder 2005; Brenna et al. 2009)—except in women of childbearing age (Burdge and Wootton 2002; Burdge et al. 2002; Smit et al. 2003; Burdge 2004; Giltay et al. 2004; Burdge and Calder 2005), while infants show a higher rate of bioconversion from exogenous sources of the precursors, LA and EPA (Sauerwald et al. 1996; Lin et al. 2010). Breastmilk supplies infants with both the preformed DHA and AA and the precursors LA and EPA (Zhang et al. 2022), that can subsequently be used by the infants themselves to biosynthesise DHA and AA.
Current evidence points to a beneficial effect of DHA and AA supplementation in approximately equal proportions in standard infant feeding formulae for infant cognitive development (Koletzko et al. 2015, 2020), showing benefits on the development of executive functions (Colombo et al. 2013, 2017; Liao et al. 2017; Carlson and Colombo 2021; Nevins et al. 2021) and activation and connectivity in cerebral cortex areas correlated with attention and cognition in term infants (Lepping et al. 2019). However, trade regulations on their presence in standard infant feeding formulae are recent. Within the European Economic Area, regulations on commercially-available formulae permitted DHA supplementation in the 2000s (Lauritzen et al. 2001, 2006), around the same time pre- and postnatal LC-PUFA supplementation became widespread (Carlson and Colombo 2021), while the first regulations mandating set quantities were promulgated almost a decade later for DHA only (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) 2014, 2015), and even later for both (Koletzko et al. 2020). Until then, commercially-available formulae were prepared with ingredients containing negligible concentration of n-3 fatty acids (Hanson and Kinsella 1981; Anderson et al. 1982; Lauritzen et al. 2001).
DHA and AA biosynthesis are heavily influenced by FADS2, a gene on chromosome eleven that is a member of the fatty acid desaturase family. FADS2 codifies enzymes present in the endoplasmic reticulum and mitochondria responsible for the insertion of double bonds in fatty acid molecules, primarily on the 6th carbon—carbon bond (Δ6 desaturase). This step is common to the metabolic pathway of both n-3 and n-6 fatty acids (Xie and Innis 2008; Lattka et al. 2010; Metherel and Bazinet 2019) and polymorphisms in FADS2 associate with different levels of DHA and AA in serum and breastmilk (Lattka et al. 2012; Xie and Innis 2008; Yeates et al. 2015; Scholtz et al. 2015; Carvalho et al. 2019; Gonzalez Casanova et al. 2021; Koletzko et al. 2011).
In 2007, Caspi et al. (2007) reported an interaction between a candidate intronic single nucleotide polymorphism (SNP) in the FADS2 gene (rs174575), breastfeeding and childhood IQ. Participants who were breastfed and carried one or more copies of the C-allele (forward strand) putatively benefitted more from breastfeeding in terms of their IQ (with a 6.4 IQ points difference between breastfed and bottle-fed children) than GG homozygotes (who exhibited no significant difference in IQ between breastfed and bottle-fed infants). The study reported an interaction of similar form in two different cohorts: 1) the Dunedin Multidisciplinary Health and Development Study, a population-based cohort from New Zealand with child IQ measured at age 7, 9, 11, and 13, and 2) the Environmental Risk (E-risk) Longitudinal Twin Study (a twin-based cohort from the UK with IQ measured at age 5). Another SNP in the region, rs1535—in linkage disequilibrium [LD] with rs174575, displayed a similar interaction in the Dunedin cohort, but not in E-risk. The authors accounted for the potential confounding effects of age, sex, self-reported ancestry, and SES. Several subsequent studies have attempted to replicate Caspi et al. (2007)’s findings, but have yielded mixed results (Steer et al. 2010; Martin et al. 2011; Groen-Blokhuis et al. 2013; Hartwig et al. 2019).
The aim of the current study was to attempt to replicate the interaction between FADS2 genotype, breastfeeding and IQ observed in Caspi et al. (2007) in the UK Biobank (Bycroft et al. 2018). Our study benefits from the availability of genotype and phenotype data on over 335,000 individuals, as well as the presence of genome-wide SNP data, which together with appropriate statistical modelling, ensures the proper control of population stratification. We investigated the effect of FADS genotype and its purported interaction with breastfeeding using both a recessive coding at FADS2 (i.e., used by many previous studies in this domain, including Caspi et al. 2007; Steer et al. 2010; Martin et al. 2011; Hartwig et al. 2019) as well as an additive coding aligned to the DHA-increasing allele (since within locus additivity appears to be the rule rather than the exception in the case of common complex quantitative traits; Hill et al. 2008; Hivert et al. 2021). Finally, we leveraged the broad phenotyping present within the UK Biobank to investigate the association between FADS2 variation and other traits that are purportedly influenced by LC-PUFA in breastmilk, including cardiometabolic phenotypes (Lattka et al. 2012) and atopy (Oddy et al. 2006; Wijga et al. 2006; Lowe et al. 2008; Singmann et al. 2010; Lattka et al. 2012).
Methods
We used the STREGA checklist when writing our report (Little et al. 2009).
UK Biobank
UK Biobank is a large-scale volunteer-based prospective study containing health and lifestyle information on over 500,000 participants living in the United Kingdom, and is detailed elsewhere (Bycroft et al. 2018). Briefly, participants were recruited between 2006 and 2010 from a pool of 9.2 million individuals living in proximity to the assessment centres, where they provided baseline sociodemographic characteristics, reported on their health and lifestyle, and were assessed through questionnaires and physical measures. The participants were also asked to provide blood samples, which were processed for genotyping and metabolite blood assays, and asked to complete a follow-up questionnaire online (from 2014). A subset of participants (n = 20,319; https://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=110003) was followed up over three in-person assessment visits (first repeat assessment [2012–2013], imaging visit [2014-ongoing], first repeat imaging visit [2019-ongoing]).
Genotyping was performed using the UKBiLEVE array (n = 49,979) and UK Biobank AxiomArray (n = 488,377). To avoid possible genotyping errors, we excluded participants from our analyses with aneuploidy (n = 651), outliers for missingness and heterozygosity (n = 968), and genetic sex—reported sex mismatch (n = 378), as defined by UK Biobank (https://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=531). We restricted our analyses to individuals who were unrelated to anyone else in the sample (up to the 3rd degree) by excluding one individual for each kinship pair (n = 80,982), or that were excluded from the kinship estimation (n = 977), as identified by the UK Biobank using the KING analysis software (Manichaikul et al. 2010; described in Bycroft et al. 2018). We further excluded all the individuals that had no imputed genotypes available (n = 15,214) and participants who withdrawn their data from the study by the time of the analysis (N = 98). For our main analyses, we restricted participants to individuals of White British ancestry, as defined by the UK Biobank, using both self-reported information and genetic PCs (n = 335,650; https://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=22006; also described in Bycroft et al. 2018). This was done in order to account for differences in allele frequency across different ancestral populations and to match previous genetic studies of FADS and breastfeeding, which have focused mainly on participants of European ancestry (Caspi et al. 2007; Steer et al. 2010; Hartwig et al. 2019). We also included the first 40 genetic PCs, as provided by UK Biobank, in order to account for residual population stratification in the sample. Finally, we derived a subset of unrelated Non-White British individuals with data on breastfeeding (n = 69,858) to investigate the effect that uncorrected population stratification might have on the presence of genetic associations. A summary flowchart of the participant selection is presented in Fig. 1.
Variant Selection
We selected three coding variants examined in previous genetic association studies of FADS2, breastfeeding and IQ (Caspi et al. 2007; Steer et al. 2010; Martin et al. 2011; Groen-Blokhuis et al. 2013; Hartwig et al. 2019). Two of the variants (rs1535 and rs174583) were directly genotyped by both UKBiLEVE and the UK Biobank AxiomArray, while the third one (rs174575) was a high-quality imputed variant (IMPUTE4 INFO score > 0.99); all the variants followed Hardy–Weinberg Equilibrium (p < 0.05). Then, we calculated the LD across variants using LDBIRD (Band et al. 2022). Summary information on the variants and their LD structure is available in Supplementary Table 1.
We extracted the allelic dosages (for rs174575) or direct genotype count (for rs1535 and rs174583) using plink 2.0 (Chang et al. 2015; Purcell and Chang 2022). We conducted statistical analyses assuming two different genetic effects on the outcomes:
-
Additive allelic effect: we coded allelic dosages so that the effect allele increased levels of DHA in blood serum. Specifically, the A allele was the effect allele for rs1535, and the C allele was the effect allele for both rs174575 and rs174583.
-
Recessive allelic effect (Caspi et al. 2007; Steer et al. 2010; Hartwig et al. 2019): we modelled the SNP under the assumption of a recessive effect of the G-allele (for rs1535 and rs174575) or the T-allele (for rs174583), coded as 1 = homozygous for the recessive allele, 0 = carrier of the other allele. This is consistent with the coding of genotypes in most previous studies of FADS2, breastfeeding and IQ (although it will most likely produce coefficients in the opposite direction to the additive coding; Caspi et al. 2007; Steer et al. 2010; Martin et al. 2011; Hartwig et al. 2019). For the imputed variant (rs174575), we used the best-guess genotype to code according to a recessive model.
We determined the DHA-increasing allele by querying openGWAS (Elsworth et al. 2020) through the function associations() available in the R package ieugwasr (https://mrcieu.github.io/ieugwasr/), selecting data based on NMR metabolomics summary results from the UK Biobank (Julkunen et al. 2022). Additionally, we investigated the association between the DHA-increasing alleles and n-3 fatty acid levels in blood serum, aligning the results to the DHA-increasing alleles. Due to the lack of data with comparable sample size for AA, we limited our analysis to LA (a precursor of AA) and n-6 fatty acids levels (Supplementary Table 2).
Breastfeeding
UK Biobank participants were asked to report on whether they were breastfed or not as a baby at baseline and during follow-up visits. As the UK Biobank is skewed towards participants in late adulthood, we restricted our analyses only to the answers gathered during the initial assessment visit (2006–2010) to minimize recall errors. This led us to identify three strata: 1) participants who answered “Yes” (up to 180,484 participants), 2) participants who answered “No” (up to 73,981 participants), and 3) participants who answered “Do not know” and “Prefer not to answer” (labelled as “Missing”; up to 81,185 participants; baseline characteristics are presented in Supplementary Table 3).
Outcomes
We investigated a range of outcomes, including cognitive measures, educational attainment, number of offspring, cardiometabolic traits, hayfever, rhinitis, and eczema. To attenuate the influence of outliers, we restricted our analysis only to participants with an outcome within 3 standard deviations of the mean. Sample size for the outcomes after exclusions and summary statistics for phenotypes and covariates are available in Supplementary Tables 4 to 8.
Cognitive Measures
The participants were asked to complete a battery of questionnaires on cognitive performance and functioning at baseline, with repeated assessments during follow-up visits (including the imaging clinics; 2014-ongoing), and an online questionnaire in 2014. Some cognitive measures were introduced at different stages of assessment; for example, part of the battery of cognitive measures was administered only to a subset of individuals at baseline (e.g., numeric memory), while other measures (e.g., matrix pattern completion) were introduced only during the imaging follow-up visit and had not been assessed at baseline. Most notably, the questionnaire for verbal-numerical reasoning differed slightly between baseline and the online follow-up, due to the addition of one question. We natural log-transformed a subset of continuous traits that appeared to be positively skewed—viz., reaction time (Lyall et al. 2016), visual memory (Lyall et al. 2016; Newby et al. 2021), and trail-making paths 1 and 2 (Hagenaars et al. 2016)—and then transformed all the variables into z-scores. We added 1 point to the visual memory variable prior to the log-transformation in order to compensate for the extreme skewness on zero values (Lyall et al. 2016), suggestive of floor effects on the measure.
When multiple data points for the cognitive measures were available, we took the first available instance, prioritising the assessment centre measure over the online measures wherever possible. Due to the different delivery mode across measures (and, in the case of the verbal-numerical reasoning scale, due to the different length of the questionnaire itself), all the models with outcomes that were administered both in the assessment centre and online (Verbal-numerical reasoning, Numeric memory, Visual memory, Trailmaking test, and Symbol digit substitution) were adjusted by the test delivery mode (0 = Assessment centre, 1 = Online).
We excluded participants who paused the assessment or who quit the assessment before completion, where information on the completion status was available.
Educational Attainment
Educational attainment was operationalised as years of education required to achieve the highest qualification reported by the participant based on the ISCED 2011 equivalence to years of education (Supplementary Table 9). This operationalization has been used previously as a measure of educational attainment (Okbay et al. 2016; Lee et al. 2018; Carter et al. 2019, 2022). We expect educational attainment to strongly correlate with cognitive development (Deary and Johnson 2010) and cognitive traits (Cornish et al. 2015).
Number of Offspring
We used number of offspring as an imperfect proxy of reproductive success, as it has been previously used to capture variants related to fertility in humans (Day et al. 2016; Barban et al. 2016; Lawn et al. 2019; Mathieson et al. 2023). We used reported number of children fathered for male participants and reported number of live births for female participants. We recoded values > 10 to 10 to minimise the impact of outliers due to recall or data entry errors (Warrington et al. 2021).
Cardiometabolic Traits
We analysed blood assay measurements of total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, and triglycerides. Measures of systolic and diastolic blood pressure (SBP and DBP, respectively) were taken at baseline and follow up, with two readings for each assessment taken with a 1-min interval. Readings were taken preferably with an automated machine and assessed manually only when the automated reading failed. We averaged the two measurements for each time point for both systolic and diastolic blood pressure. We selected the earliest available automatic measurement, where available; otherwise, we used the earliest available manual measurement. We accounted for blood pressure medication use by adding 15 mmHg to systolic blood pressure when the participants were under blood pressure lowering medications, and 10 mmHg for diastolic blood pressure (Wang et al. 2022). Body mass index (BMI; \(kg/{m}^{2}\)) was calculated by UK Biobank from the height (m) and weight (kg) measures taken at baseline from the assessment centres.
We also investigated four cardiometabolic diseases using the self-reported diagnoses from the assessment centre’s touchscreen health questionnaires, including angina pectoris, myocardial infarction, stroke, and type-II diabetes. Participants were coded as type-II diabetes cases if they replied “Yes” to the question "Has a doctor ever told you that you have diabetes?" and “No” to "Did you start insulin within one year of your diagnosis of diabetes?" at any instance. The age covariate was set to the minimum age when they reported the diagnosis to the UK Biobank or the maximum age without reporting a diagnosis. Participants who responded “Prefer not to answer” were set to missing.
Hayfever, Allergic Rhinitis, or Eczema
Finally, we investigated the relationship between FADS2 and breastfeeding on hayfever, allergic rhinitis, or eczema from the assessment centre’s touchscreen questionnaire. We could not differentiate between the three conditions as no further question was asked of the UK Biobank participants to distinguish between them. To control for possible cohort effects in reporting atopic dermatitis, we used date of birth as a time covariate. Participants who responded “Prefer not to answer” or did not answer the question were set to missing.
Statistical Modelling
To test for the association between breastfeeding and our outcomes, we fit:
1. A univariate model
2. A model adjusting for the additive effect of age and sex:
3. A model adjusting for the additive effect of age, sex, and Townsend deprivation index (TDI):
4. A model adjusting for the additive effect of age, sex, TDI, and 40 genetic PCs:
where for every participant \(i\), \(Y\) is the outcome of interest, \({\beta }_{0}\) is the intercept, \(BF\) is the participant’s breastfeeding status (where 0 = bottle-fed, 1 = breastfed), with effect \({\beta }_{BF}\) on the outcome, \(age\) is the participant’s age at assessment, with effect \({\beta }_{age}\), \(sex\) is the participant’s sex (where 0 = female, 1 = male), with effect \({\beta }_{sex}\), \(TDI\) is the participant’s TDI, with effect \({\beta }_{TDI}\), \({P{C}_{k}}_{i}\) is the value of the \(k\) th genetic PC for participant \(i\), with effect \({\beta }_{P{C}_{k}}\), and \({\varepsilon }_{i}\) is a random error term.
To test for the FADS-by-breastfeeding interaction, we fit.
1. An unadjusted interaction model:
2. A model adjusting for the additive effect of age and sex:
3. A model adjusting for the additive effect of age, sex, and 40 genetic PCs:
4. A model adjusting for the additive effect of age, sex, and PCs, and all the pairwise interactions across covariates (Keller 2014):
where \(G\) is the participant’s genotype, modelled using either a recessive coding (Caspi et al. 2007; Steer et al. 2010; Hartwig et al. 2019) or an additive coding, counting the DHA-increasing allele (i.e., A for rs1535, C for rs174575, C for rs174583), with effect \({\beta }_{G}\) on the outcome, \({\beta }_{G\times BF}\) is the effect of the gene-by-environment interaction between genotype \(G\) and breastfeeding status \(BF\), \({\beta }_{G\times age}\) is the effect of the interaction between \(G\) and \(age\), \({\beta }_{G\times sex}\) is the effect of the interaction between \(G\) and \(sex\), \({\beta }_{age\times sex}\) is the effect of the interaction between \(age\) and \(sex\), is the effect of the interaction between \(age\) and \(BF\), \({\beta }_{sex\times BF}\) is the effect of the interaction between \(sex\) and \(BF\), \({\beta }_{G\times P{C}_{k}}\) is the effect of the interaction between \(G\) and the \(k\) th \(PC\), \({\beta }_{BF\times P{C}_{k}}\) is the effect of the interaction between \(BF\) and the \(k\) th \(PC\), \({\beta }_{age\times P{C}_{k}}\) is the effect of the interaction between \(age\) and the \(k\) th \(PC\), and \({\beta }_{sex\times P{C}_{k}}\) is the effect of the interaction between \(age\) and the \(k\) th \(PC\).
To better visualize the results, we also fit the above models to each stratum of breastfeeding (breastfed, bottlefed, or missing) without the interaction term.
For all analyses, we fit linear regression if the outcome was continuous, and logistic regression if the outcome was binary. Additionally, if the same cognitive measure was assessed both in person (at the assessment centre or during the imaging visits) and online, we added an additional covariate to all models to adjust for possible differences due to the delivery format and, in the case of verbal-numerical reasoning, a different number of questions in the questionnaire.
We restricted our analyses to participants with complete data on exposures and outcome in the given model.
Results
Phenotypic Associations
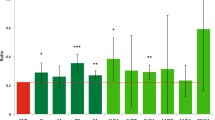
Consistent with many previous studies (Michaelsen et al. 2009; Horta et al. 2018), being breastfed as a baby was associated (p < 0.05) with improved cognitive function after controlling for covariates—particularly age and sex (Fig. 2 and Supplementary Table 10 and 11). This included tests for verbal-numerical reasoning, reaction time, numeric memory, paired associate learning, matrix pattern completion, symbol-digit substitution, tower rearranging and trail-making path tests. We also observed evidence of an observational association between being breastfed and an increase in years of schooling and a decrease in number of offspring (after controlling for covariates; Supplementary Table 11). In the case of the cardiometabolic diseases, being breastfed was associated (p < 0.05) with decreased risk of angina and heart attack, but not stroke or type-II diabetes after controlling for covariates (Supplementary Table 12). Being breastfed was associated with increased LDL, HDL and total cholesterol, and decreased triglycerides, BMI, and blood pressure (Supplementary Table 11). Finally, breastfeeding was observationally associated with increased risk of hayfever, rhinitis, and eczema (Supplementary Table 12).
Association between breastfeeding and cognitive, noncognitive quantitative, and binary outcomes. Results are presented as change in z-score of the outcome (for quantitative traits) or change in log odds of being affected by the outcome when individuals are breastfed. All models were adjusted for age, sex, genetic principal components, and Townsend deprivation index. Error bars represent 95% confidence intervals
Association Between FADS2 Variants and Fatty Acid Levels
We confirmed that all three FADS variants showed strong evidence of association with serum fatty acid levels in the expected directions (i.e., the mediating biomarkers through which breast feeding is thought to exert its beneficial effects; Supplementary Table 2) (Caspi et al. 2007; Hartwig et al. 2019; Borges et al. 2022). All the variants were associated with DHA blood serum levels and with the ratio of DHA to total fatty acid levels. Alleles associated with an increase in DHA blood levels were associated with decreased LA blood serum levels and the ratio of LA to total fatty acids. All the DHA-increasing alleles were also associated with increased amount of PUFA, n-3, and n-6 fatty acids in the blood serum and their ratio to total fatty acids, and decreased n-6:n-3 ratio.
FADS2 Main Effect and FADS2-by-Breastfeeding Interaction
All three FADS2 variants were in strong LD with one another (\({r}^{2}\ge 0.68\); Supplementary Table 1). We found little evidence for a main effect of rs174575 on verbal-numerical reasoning in the UK Biobank, when modelling rs174575 as recessive for the G allele as Caspi et al. (Caspi et al. 2007) did (Fig. 3). However, when modelling rs174575 under an additive genetic model (Fig. 4a), additional copies of the DHA-increasing (C) allele were instead associated with decreased verbal-numerical reasoning (Fig. 4, Supplementary Table 13).We found no evidence of interaction between rs174575 and breastfeeding on verbal-numerical reasoning, regardless of the covariate adjustment strategy (Supplementary Fig. 1), both assuming a recessive model (Fig. 3) or an additive model (Fig. 4a and Supplementary Fig. 1). The other SNPs showed similar results (Supplementary Tables 13 to 16). However, when extending the sample to non-White British participants, we observed evidence for a small interaction in verbal-numerical reasoning when the model did not account for PCs (Supplementary Fig. 4 and Supplementary Table 17).
Effect estimates for the rs174575 main effect on verbal-numerical reasoning in the UK Biobank and in previous studies, and estimates of its interaction with breastfeeding. E-risk: Environmental risk study from Caspi et al. (2007); Dunedin: Dunedin Multidisciplinary Health and Development Study from Caspi et al. (2007); ALSPAC: Avon Longitudinal Study of Parents and Children from Steer et al. (2010); UK Biobank: effect estimate derived by the present study on UK Biobank. (1) unadjusted; (2) adjusted for age and sex; (3) adjusted for age, sex, and principal components; (4) adjusted for age, sex, principal components, and pairwise interactions across covariates (Keller 2014). Hartwig et al. (2019) did not report an estimate of the main effect of rs174575. We derived the regression coefficients for Caspi et al. (2007) from the mean and standard deviation of IQ for each subgroup reported in Table 1. The regression coefficients are expressed in change in standard deviations of Verbal-Numerical reasoning for change in genotype of rs174575 (modelled as a recessive effect of the C allele) for the main effect of rs174575, and in change of standard deviations of verbal-numerical reasoning for homozygous of the recessive alleles and breastfed individuals for the interaction term. Note that the large standard errors around the recessive main effect estimate in UK Biobank (4) are due to the covariate interaction terms correlating strongly with the recessive SNP coding. Error bars represent 95% confidence intervals
Results from the association analysis between rs174575 and all the outcomes by breastfeeding status, assuming an additive genetic effect of the DHA-increasing allele. The results are shown for rs174575 adjusting for age, sex, and principal components. a results for the cognitive traits; b results for the non-cognitive continuous traits; c results for the binary traits (affected-unaffected). The results are expressed in change of standard deviations of the phenotype per increase in effect allele copies (for a and b) or change in natural log odds of being affected by the phenotype per increase in effect allele copies (for c). Error bars represent 95% confidence intervals
The DHA-increasing alleles at the three SNPs showed strong evidence of association with increased total cholesterol, LDL cholesterol, HDL cholesterol and number of offspring, and decreased triglycerides, BMI and blood pressure. Interestingly, assuming a recessive model at these same SNPs of the sort espoused by Caspi et al. (2007), attenuated the evidence for association at most of the loci/phenotypes by several orders of magnitude. Similar, to the cognitive traits, the magnitude of the associations did not appear to be affected by breastfeeding status (Supplementary Table 13 and 15).
Discussion
We failed to replicate an interaction between FADS2 genotype and self-reported breastfeeding status on any of the measures of cognitive functioning we investigated, or any of the other traits we examined. Our study constitutes the largest attempt to replicate the Caspi et al. (2007) interaction findings to date, and our results are similar to most, but not all previous attempts at replicating this interaction. A previous study by Steer et al. (Steer et al. 2010) also reported an interaction between breastfeeding and FADS2 genotype on IQ in children from the Avon Longitudinal Study of Parents and Children (ALSPAC), a population-based longitudinal cohort with similar allele frequencies to E-risk and using the same family of IQ tests (Wechsler Intelligence Scale). However, the form of the reported interaction in Steer et al. (2010) was different to that of Caspi et al. (2007): Steer et al. (2010) found a positive effect of breastfeeding on IQ in individuals homozygous for the G allele at the rs174575 variant, but not for carriers of the C allele. The two studies further differed in the way of reporting breastfeeding (i.e., prospectively in ALSPAC; retrospectively at age 2 or 3 in Dunedin and E-risk) and age at IQ testing (a combined measure of age 7, 9, 11, and 13 in Dunedin; 5 in E-risk; age 8 in ALSPAC).
An Australian twin study by Martin et al. (2011) also failed to replicate the FADS2-by-breastfeeding interaction on IQ using a measure of cognitive functioning at age 16 and controlling for maternal SES. They also extended the analysis to rs174583, a non-palindromic SNP (T-C) in LD with the palindromic rs174575 (C-G), alongside the two previous variants, with similar results. Groen-Blokhuis et al. (2013) investigated the same interaction for rs1535 ad 174,575 on IQ measures at age 5, 7, 10, 12, and 18 (assessed with different scales), educational attainment, and maternally rated overactivity and attention problems in a different sample of twins from the Netherlands. Despite finding a small improvement in educational attainment, overactive behaviour, and IQ in breastfed twins compared to bottle-fed twins, they did not provide any evidence of an interaction between rs174575 and breastfeeding on childhood IQ or any of the other traits.
The largest and most comprehensive attempt at replicating the Caspi et al. (2007) interaction was a study by Hartwig et al. (2019) (12,077–13,202 individuals) who conducted a pre-registered (Hartwig et al. 2016) meta-analysis of studies, including the ALSPAC and Dunedin cohorts (Caspi et al. 2007; Steer et al. 2010). The study excluded twin-based cohorts and limited the analysis to individuals of European ancestry, controlling for ancestry-informative PCs where possible, but failed to provide evidence of an interaction between breastfeeding and rs174575 on childhood IQ.
Most of these previous studies have been small, which historically has been typical of candidate gene studies (Colhoun et al. 2003; Zondervan and Cardon 2007; Okbay and Rietveld 2015; Duncan et al. 2019). Our results seem to align with the findings of Hartwig and colleagues (2019), which constitutes the best evidence prior to this study due to a rigorous pre-registered protocol, control for ancestry informative PCs where possible, and a large sample size (Hartwig et al. 2016). There could be several reasons why a gene-by-environment interaction was observed in some of the previous studies—viz., Caspi et al. (2007) and Steer et al. (2010)—but not others. A common criticism of candidate gene association studies is lack of sufficient control for population stratification (Sullivan 2007; Okbay and Rietveld 2015). Failure to account for latent underlying differences in ancestry may induce spurious associations between traits and genetic variants that are functionally unrelated to the traits of interest (Cardon and Palmer 2003). The Caspi et al. (2007) and Steer et al. (2010) studies attempted to control for population stratification by restricting their samples to a subset of homogeneous individuals, based on self-reported ancestry. However, this approach is often not sufficient to control for fine-scale genetic differences between populations and so it is likely that residual stratification was present. The use of ancestry-informative PCs as covariates is a common and effective way of controlling for population stratification in genetic association studies, as the PCs more accurately model individuals’ ancestry and capture more nuanced differences in latent population substructure beyond the major ancestral groups, thereby reducing the chance of spurious findings (Price et al. 2006). In the present study, we accounted for population stratification in two ways: by using an ancestrally-homogeneous subset of UK Biobank individuals (as assessed by self-reported ancestry and scores on ancestrally-informative PCs; Bycroft et al. 2018), and by fitting the PCs themselves as covariates. Furthermore, when repeating the analysis for a larger, less ancestrally homogeneous sample of individuals (Supplementary Fig. 4), we detected a weak genotype-by-breastfeeding interaction effect for rs1535 when not controlling for ancestry informative PCs, but this interaction disappeared after controlling for PCs. Our finding aligns with Hartwig et al. (2019), where adjustment for PCs explained a substantial portion of the genotype-by-breastfeeding interaction in studies where this information was available. The implication is that previous positive findings may have been a result of inadequate control for population stratification, rather than genuine SNP-by-breastfeeding interaction effects.
Gene-by-environment interaction studies have also been criticised for not accounting for gene-by-covariate, environment-by-covariate, and covariate-by-covariate interactions. First suggested by Yzerbyt et al. (2004) and highlighted by Keller (2014) in the context of interaction studies with candidate variants, not accounting for pairwise interactions across covariates can bias the effect estimate for the interaction term. In the context of the present study, we performed the adjustment for the rs174575 variant, but we did not observe a substantial impact on the point estimates (which were close to the null). However, the standard error for the main effect estimate of the SNP drastically changed when fitting all the pairwise interactions across covariates (presumably because of high correlation between the recessive coding and the additional interaction terms in the model; Fig. 3).
Finally, both Caspi et al. (2007) and Steer et al. (2010) fit different socioeconomic and physical covariates in their models, including SES (in both studies), maternal education, low birthweight, pre-term gestation, home environment, and parenting (in Steer et al. 2010). The use of heritable covariates to investigate genetic associations has the potential to bias the association or, in absence of a causal link, induce a spurious association, depending on the trait’s latent causal structure (Aschard et al. 2015). Thus, it is possible that the interaction detected by the previous studies could have been partly or fully induced due to conditioning on a collider (Cole et al. 2010). In the context of the present study, we limited our covariate adjustments to age, sex, and PCs.
We provide some evidence for a main (additive) effect of rs1535, rs174575, and rs174583 on cognitive development, with all three SNPs showing an effect on the most commonly used proxy for cognitive development in UK Biobank (Lyall et al. 2016; Savage et al. 2018; Hartwig et al. 2019; Cox et al. 2019; de Nooij et al. 2020), verbal-numerical reasoning. However, the direction of effect for verbal-numerical reasoning is opposite to what we would expect: since our analyses are aligned to the DHA-increasing allele, an increase in the effect allele count should lead to an increase in availability of DHA in breastmilk, and hypothetically an increase in cognitive ability. However, our results involve an inverse association. To determine whether this discrepancy reflects a genuine association in the opposite direction, we compared our results with a previous GWAS meta-analysis on intelligence (N = 269,867; Savage et al. 2018), which included unrelated participants from UK Biobank who also responded to the questionnaire on verbal-numerical reasoning (N = 195,653; also present in our study). In the case of a genuine genetic association, we would expect the increase in sample size to improve our evidence of an association and the direction of effect to align between the two samples. While the direction of effect for all three SNPs aligns with what we observed in the present study, the additional cohorts integrated in the meta-analysis improved the evidence of an association for only two SNPs (rs1535 and rs174575), while the p-value for rs174583 remained roughly unchanged— \(p=5.66\times {10}^{-5}\) in Savage et al. (2018), \(p=4.45\times {10}^{-5}\) in the present study (Supplementary Table 21). Due to the increase in sample size in the meta-analysis (N = 74,214) and the strong LD with rs1535 (\({r}^{2}=0.96\)), we would expect rs174583 to benefit from the inclusion of additional cohorts, hinting at the possibility of a false positive in the UK Biobank.
Another possibility is that the SNPs in FADS2 are capturing an independent signal from a causal variant within the region. When looking at the LD structure surrounding FADS2, the SNPs appear to be independent from the nearest genome-wide significant top hit (rs77128898), making it unlikely they are tagging signals from that region. On the other hand, as the region immediately surrounding the variants in FADS2 hosts several other genes, the SNPs in FADS2 may still be tagging an independent signal not originating from FADS2, namely: MYRF, a transcription factor involved in myelination of the central nervous system; FADS1, another desaturase part of the DHA and AA biosynthesis; MIR611 and FEN1, involved in the alteration of miRNA and Okazaki fragments, respectively; and TMEM258, involved in protein N-linked glycosylation (Supplementary Fig. 5). Nevertheless, inference regarding the causal mechanisms across the region is a difficult process (often involving fine-mapping, a statistical procedure attempting to determine the source of signal within a genome-wide significant region; Schaid et al. 2018) and strong evidence of the association of the trait in the region. Further investigation of the region surrounding FADS2 is required to elucidate its role in cognitive development.
Our results from the UK Biobank align with much of the current literature suggesting a positive effect of breastfeeding on cognitive traits, after controlling for covariates (Horta et al. 2015). Nevertheless, our analyses are based upon observational data. Observational association studies of breastfeeding are typically affected by residual confounding, selection bias, and reverse causation, as has been noted in previous studies involving IQ, where maternal IQ and socioeconomic status may influence the likelihood of being breastfed, or reporting to be breastfed (Michaelsen et al. 2009; Walfisch et al. 2013; Cornish et al. 2015).
While some quasi-experimental designs robust to reverse causation have also observed a positive effect of breastfeeding on IQ (Kramer et al. 2008; Denny and Doyle 2010), the breastfeeding literature is likely subject to a considerable publication bias (Ritchie 2017), inflating the ratio of reported positive to null findings. Ultimately, more studies are necessary to dissect the causal relationship between breastfeeding and cognition.
Likewise, the relationship between breastfeeding and the development of atopy is unclear. Previous studies have reported protective (Yang et al. 2009), predisposing (Mathieson et al. 2023) or no effect (Lin et al. 2020) of breastfeeding on risk of atopy as well widespread heterogeneity in estimated effect sizes across studies. Reverse causation is also a likely problem in the interpretation of these many of these studies in that early signs of atopic allergy may result in infants being breastfed/being breastfed for longer (Lowe et al. 2008; Lodge et al. 2008; Kusunoki et al. 2010; Bigman 2020; Peters et al. 2021). In the UK Biobank, we found that breastfeeding was protective for atopy in unadjusted analyses, but risk predisposing after conditioning on covariates including age, sex, genetic principal components and total deprivation index. It is unclear what this pattern of results reflects, however, we caution against their over-interpretation because of the well described problems of selection/collider bias in the UK Biobank (Munafò et al. 2018), and the fact that the data is retrospective, coarse (i.e., does not differentiate between different atopic conditions) and based on self-report.Several limitations of the present study arise from the use of self-reported breastfeeding status. Recollection of their own breastfeeding status by the UK Biobank participants is susceptible to self-report bias, as the recall is likely dependent on the information being passed on by their mother and/or other relatives. The UK Biobank participants’ year of birth (1934–1971) corresponds to a period generally regarded as having low breastfeeding initiation and cultural taboos related to breastfeeding practices in the UK (Thorley 2019). While systematic collection of data on breastfeeding practices succeeded that time—the Infant Feeding Survey, the longest-running survey on infant feeding practices in the country, started only in 1975 (Martin 1978), data from other countries generally show a steady decline in breastfeeding from 1930 to 1970s, when infant formula became the prevalent way of feeding infants (WHO Collaborative Study on Breast-Feeding and Organization 1981), and indeed the UK retains a low uptake of breastfeeding to this day (Simpson et al. 2019). Conversely, around ¾ of the UK Biobank participants with data on the question reported being breastfed as infants. Due to the promotion of breastfeeding as the “healthy” way of feeding infants, together with a sampling skewed more towards healthier, older, higher educated, less deprived, and female individuals (i.e., more likely to have been exposed to breastfeeding promotion campaigns; Fry et al. 2017; Batty et al. 2020; Stamatakis et al. 2021), we would expect generally higher rates of breastfeeding than the general population.
However, this skewness could also partially be due to non-differential misclassification bias (Whitcomb and Naimi 2020): participants would be more likely to have answered they were breastfed than bottle fed, when in doubt. As we expect these variants to show an effect in the breastfed individuals, this could bias our estimates towards the null.
Other potential issues that could impact our estimates are due to the varying duration of breastfeeding across participants. Early weaning well before the currently recommended six months of exclusive breastfeeding (World Health Organization 2003) has been reported to be common practice before 1970 (Department of Health and Social Security 1974), and it is still occurring in the UK to this day (Health and Social Care Information Centre, IFF Research 2012). As we expect a change in the concentration of DHA in breastmilk according to the levels of FADS2 genotype only within the breastfed group, if an interaction were present, a shorter breastfeeding duration would attenuate the effect of DHA on the outcome.
The quality of cognitive measures in the UK Biobank presents further challenges to our findings. The previous literature describing the effect of breast feeding on IQ is heterogeneous in terms of scales used for IQ assessments and age at assessment, but it is predominantly composed of validated scales of child IQ. In contrast, the cognitive measures available in the UK Biobank were taken in mid- to late adulthood and used as a proxy for early childhood cognitive development. While absolute cognitive performance declines after early adulthood (Salthouse 2009; Deary et al. 2009), intelligence does show rank-order stability over time (i.e., an individual’s position according to a reference population; Gow et al. 2011; Deary 2014), which is the rationale behind the widespread use of standardised IQ scores. Thus, by assuming temporal stability and accounting for age and cohort differences (Cornelis et al. 2019), adult cognitive performance can be used as a proxy for childhood intelligence to observe differences across subgroups, although with different temporal stability across ranks (Whitaker 2008; Schneider et al. 2014).
Despite being mostly based on established psychometric measurements, the adoption of non-validated tests without data on their standardisation poses difficulties in the interpretation of our results. In particular, it is difficult to compare the UK Biobank participants’ performance with the general population. As the effect of a supposed breastfeeding-by-FADS2 interaction might not be uniform across the whole population, the lack of standardisation prevents inferences drawn from quantile-based subgroup analyses. Further, the relative homogeneity in stability of the UK Biobank cognitive tests (Cornelis et al. 2019), together with the known sampling biases (Fry et al. 2017), suggests the lower ranks of cognitive abilities might be underrepresented, which might be also affected differently by the gene-by-breastfeeding interaction.
Finally, some studies (Hagenaars et al. 2016; Fawns-Ritchie and Deary 2020) have noted that the most-commonly used measure used to proxy intelligence in the UK Biobank—labelled fluid intelligence by UK Biobank, here and in the literature often named verbal-numerical reasoning (Lyall et al. 2016; Cox et al. 2019; de Nooij et al. 2020; Fawns-Ritchie and Deary 2020)—might capture a blend of crystallised and fluid abilities. Following Hebb and Cattel’s paradigm on intelligence (Brown 2016; Kent 2017), fluid intelligence represents what is commonly known as “intelligence”, a task-independent cognitive performance, while crystallised abilities reflect skills acquired through learning and practice over time (i.e., developed through the investment of fluid intelligence in new tasks). This distinction can be also observed in the underlying biology of the trait, with fluid and crystallised skills showing different genetic associations and pathways (Christoforou et al. 2014); verbal (crystallised) and non-verbal (fluid) skills have been also observed to have different topological correlates during brain development (Ramsden et al. 2011). As the FADS2-by-breastfeeding interaction we have investigated should influence predominantly fluid skills, with a lesser impact on crystallised skills in later life, capturing crystallised abilities could attenuate the effect of a possible interaction, reducing power to detect an effect with our current sample size.
Our statistical analyses involved complete case analyses. Whereas all participants had information on genotype, age, sex and genome-wide genetic principal components, there was non-trivial amounts of missingness with respect to breastfeeding status and many of the outcome variables examined in this manuscript. The reasons for these missing data are unclear but are unlikely to be related to FADS2 status. The main effects analyses (i.e., between the FADS2 variant and the various outcomes) were stratified on breastfeeding status (i.e., including those who did not report their breastfeeding status as a separate category) and effect size estimates for the FADS2 variant were similar across strata. However, it is conceivable that at least some of the outcomes were “missing not at random” (i.e., the reason for the missingness was related to some of the outcomes themselves which were missing and/or other variables that were not captured as part of the UK Biobank assessment). For example, if missingness were caused by IQ (e.g., more intelligent individuals were more likely to complete IQ related assessments), then complete case analyses would result in biased estimates of any genuine FADS2 association and its interaction. Importantly, however, this reason for missingness should not result in increased type-I error in the absence of genuine FADS2 association/interaction with breastfeeding, and so is unlikely to explain the absence of breastfeeding SNP interactions in the present study. The use of techniques such as multiple imputation, in place of a complete case analysis, may further bias our results as they often assume data that is “missing at random” (Hughes et al. 2019).
Despite benefitting from the largest sample size to date, our study does not provide evidence for a FADS2-by-breastfeeding interaction on any of the traits we investigated, after strict control for population structure. Our findings align with smaller studies that have also failed to detect the interaction after controlling for population structure through PCs (Hartwig et al. 2019). We provide evidence for a small main effect of the FADS2 variants (rs1535, rs174575, rs174583) on a subset of our traits (viz., the verbal-numerical reasoning scale for cognitive traits, number of offspring, and lipid measures).
Data Availability
The UK Biobank data described in the manuscript is available to all bona fide research upon application and approval. Code book and analytic code will be made publicly and freely available without restrictions at https://github.com/GiulioCentorame/FADS-by-breastfeeding/
Abbreviations
- AA:
-
Arachidonic acid
- ALSPAC:
-
Avon Longitudinal Study of Parents and Children
- DBP:
-
Diastolic blood pressure
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaneoic acid
- E-risk:
-
Environmental Risk Longitudinal Twin Study
- GWAS:
-
Genome-wide association study
- HDL:
-
High-density lipoprotein
- LA:
-
Linoleic acid
- LC-PUFA:
-
Long-chain polyunsaturated fatty acids
- LD:
-
Linkage disequilibrium
- LDL:
-
Low-density lipoprotein
- PC:
-
Principal component
- PUFA:
-
Polyunsaturared fatty acids
- SBP:
-
Systolic blood pressure
- SES:
-
Socioeconomic status
- SNP:
-
Single nucleotide polymorphism
- TDI:
-
Townsend deprivation index
References
Anderson SA, Chinn HI, Fisher KD (1982) History and current status of infant formulas. Am J Clin Nutr 35:381–397. https://doi.org/10.1093/ajcn/35.2.381
Aschard H, Vilhjálmsson BJ, Joshi AD et al (2015) Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am J Hum Genet 96:329–339. https://doi.org/10.1016/j.ajhg.2014.12.021
Auton A, Abecasis GR, Altshuler DM et al (2015) A global reference for human genetic variation. Nature 526:68–74. https://doi.org/10.1038/nature15393
Band G, Leffler EM, Jallow M et al (2022) Malaria protection due to sickle haemoglobin depends on parasite genotype. Nature 602:106–111. https://doi.org/10.1038/s41586-021-04288-3
Barban N, Jansen R, de Vlaming R et al (2016) Genome-wide analysis identifies 12 loci influencing human reproductive behavior. Nat Genet 48:1462–1472. https://doi.org/10.1038/ng.3698
Batty GD, Gale CR, Kivimäki M et al (2020) Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ 368:m131. https://doi.org/10.1136/bmj.m131
Bigman G (2020) The Relationship of Breastfeeding and Infant Eczema: The Role of Reverse Causation. Breastfeed Med 15:114–116. https://doi.org/10.1089/bfm.2019.0269
Binns C, Lee M, Low WY (2016) The long-term public health benefits of breastfeeding. Asia Pac J Public Health 28:7–14. https://doi.org/10.1177/1010539515624964
Borges MC, Haycock PC, Zheng J et al (2022) Role of circulating polyunsaturated fatty acids on cardiovascular diseases risk: analysis using Mendelian randomization and fatty acid genetic association data from over 114,000 UK Biobank participants. BMC Med 20:210. https://doi.org/10.1186/s12916-022-02399-w
Brenna JT, Diau G-Y (2007) The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fatty Acids 77:247–250. https://doi.org/10.1016/j.plefa.2007.10.016
Brenna JT, Salem N, Sinclair AJ, Cunnane SC (2009) α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids 80:85–91. https://doi.org/10.1016/j.plefa.2009.01.004
Brown RE (2016) Hebb and cattell: the genesis of the theory of fluid and crystallized intelligence. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2016.00606
Burdge G (2004) Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 7:137–144. https://doi.org/10.1097/00075197-200403000-00006
Burdge GC, Calder PC (2005) Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 45:581–597. https://doi.org/10.1051/rnd:2005047
Burdge GC, Wootton SA (2002) Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 88:411–420. https://doi.org/10.1079/BJN2002689
Burdge GC, Jones AE, Wootton SA (2002) Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men*. Br J Nutr 88:355–363. https://doi.org/10.1079/BJN2002662
Bycroft C, Freeman C, Petkova D et al (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562:203–209. https://doi.org/10.1038/s41586-018-0579-z
Cardon LR, Palmer LJ (2003) Population stratification and spurious allelic association. The Lancet 361:598–604. https://doi.org/10.1016/S0140-6736(03)12520-2
Carlson SE, Colombo J (2021) DHA and cognitive development. J Nutr 151:3265–3266. https://doi.org/10.1093/jn/nxab299
Carter AR, Gill D, Davies NM et al (2019) Understanding the consequences of education inequality on cardiovascular disease: mendelian randomisation study. BMJ 365:l1855. https://doi.org/10.1136/bmj.l1855
Carter AR, Harrison S, Gill D et al (2022) Educational attainment as a modifier for the effect of polygenic scores for cardiovascular risk factors: cross-sectional and prospective analysis of UK Biobank. Int J Epidemiol 51:885–897. https://doi.org/10.1093/ije/dyac002
Carvalho GQ, Pereira-Santos M, Marcon LD et al (2019) Maternal polymorphisms in the FADS1 and FADS2 genes modify the association between PUFA ingestion and plasma concentrations of omega-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 150:38–46. https://doi.org/10.1016/j.plefa.2019.09.004
Caspi A, Williams B, Kim-Cohen J et al (2007) Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc Natl Acad Sci U S A 104(18860):18865. https://doi.org/10.1073/pnas.0704292104
Chang CC, Chow CC, Tellier LC et al (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. https://doi.org/10.1186/s13742-015-0047-8
Christoforou A, Espeseth T, Davies G et al (2014) GWAS-based pathway analysis differentiates between fluid and crystallized intelligence. Genes Brain Behav 13:663–674. https://doi.org/10.1111/gbb.12152
Cole SR, Platt RW, Schisterman EF et al (2010) Illustrating bias due to conditioning on a collider. Int J Epidemiol 39:417–420. https://doi.org/10.1093/ije/dyp334
Colhoun HM, McKeigue PM, Smith GD (2003) Problems of reporting genetic associations with complex outcomes. The Lancet 361:865–872. https://doi.org/10.1016/S0140-6736(03)12715-8
Colombo J, Carlson SE, Cheatham CL et al (2013) Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr 98:403–412. https://doi.org/10.3945/ajcn.112.040766
Colombo J, Jill Shaddy D, Kerling EH et al (2017) Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot Essent Fatty Acids 121:52–56. https://doi.org/10.1016/j.plefa.2017.05.005
(2015) Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding (Text with EEA relevance)
Cornelis MC, Wang Y, Holland T et al (2019) Age and cognitive decline in the UK Biobank. PLoS ONE 14:e0213948. https://doi.org/10.1371/journal.pone.0213948
Cornish RP, Tilling K, Boyd A et al (2015) Using linked educational attainment data to reduce bias due to missing outcome data in estimates of the association between the duration of breastfeeding and IQ at 15 years. Int J Epidemiol 44:937–945. https://doi.org/10.1093/ije/dyv035
Cox SR, Ritchie SJ, Fawns-Ritchie C et al (2019) Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence 76:101376. https://doi.org/10.1016/j.intell.2019.101376
Day FR, Helgason H, Chasman DI et al (2016) Physical and neurobehavioral determinants of reproductive onset and success. Nat Genet 48:617–623. https://doi.org/10.1038/ng.3551
de Nooij L, Harris MA, Adams MJ et al (2020) Cognitive functioning and lifetime major depressive disorder in UK Biobank. Eur Psychiatry 63:e28. https://doi.org/10.1192/j.eurpsy.2020.24
Deary IJ (2014) The Stability of Intelligence From Childhood to Old Age. Curr Dir Psychol Sci 23:239–245. https://doi.org/10.1177/0963721414536905
Deary IJ, Johnson W (2010) Intelligence and education: causal perceptions drive analytic processes and therefore conclusions. Int J Epidemiol 39:1362–1369. https://doi.org/10.1093/ije/dyq072
Deary IJ, Corley J, Gow AJ et al (2009) Age-associated cognitive decline. Br Med Bull 92:135–152. https://doi.org/10.1093/bmb/ldp033
Denny K, Doyle O (2010) The causal effect of breastfeeding on children’s cognitive development: A quasi-experimental design. UCD Centre for Economic Research Working Paper Series
Department of Health and Social Security (1974) Present-day practice in infant feeding: Report of a working party of thhe panel on child nutrition, committee on medical aspects of food policy. H.M.S.O, London
(2006) Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC Text with EEA relevance
Duncan LE, Ostacher M, Ballon J (2019) How genome-wide association studies (GWAS) made traditional candidate gene studies obsolete. Neuropsychopharmacology 44:1518–1523. https://doi.org/10.1038/s41386-019-0389-5
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2014) Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J 12:3760. https://doi.org/10.2903/j.efsa.2014.3760
Elsworth B, Lyon M, Alexander T, et al (2020) The MRC IEU OpenGWAS data infrastructure. 2020.08.10.244293
Falkenburger BH, Jensen JB, Dickson EJ et al (2010) Phosphoinositides: lipid regulators of membrane proteins. J Physiol 588:3179–3185. https://doi.org/10.1113/jphysiol.2010.192153
Fawns-Ritchie C, Deary IJ (2020) Reliability and validity of the UK Biobank cognitive tests. PLoS ONE 15:e0231627. https://doi.org/10.1371/journal.pone.0231627
Flicek P, Amode MR, Barrell D et al (2014) Ensembl 2014. Nucleic Acids Res 42:D749–D755. https://doi.org/10.1093/nar/gkt1196
Forsyth S, Gautier S, Salem N Jr (2016) Global Estimates of Dietary Intake of Docosahexaenoic Acid and Arachidonic Acid in Developing and Developed Countries. Ann Nutr Metab 68:258–267. https://doi.org/10.1159/000446855
Fry A, Littlejohns TJ, Sudlow C et al (2017) Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol 186:1026–1034. https://doi.org/10.1093/aje/kwx246
Giltay EJ, Gooren LJG, Toorians AWFT et al (2004) Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr 80:1167–1174. https://doi.org/10.1093/ajcn/80.5.1167
Gonzalez Casanova I, Schoen M, Tandon S et al (2021) Maternal FADS2 single nucleotide polymorphism modified the impact of prenatal docosahexaenoic acid (DHA) supplementation on child neurodevelopment at 5 years: Follow-up of a randomized clinical trial. Clin Nutr Edinb Scotl 40:5339–5345. https://doi.org/10.1016/j.clnu.2021.08.026
Gow AJ, Johnson W, Pattie A et al (2011) Stability and change in intelligence from age 11 to ages 70, 79, and 87: The Lothian Birth Cohorts of 1921 and 1936. Psychol Aging 26:232–240. https://doi.org/10.1037/a0021072
Groen-Blokhuis MM, Franić S, van Beijsterveldt CEM et al (2013) A prospective study of the effects of breastfeeding and FADS2 polymorphisms on cognition and hyperactivity/attention problems. Am J Med Genet Part B Neuropsychiatr Genet off Publ Int Soc Psychiatr Genet 162B:457–465. https://doi.org/10.1002/ajmg.b.32175
Hagenaars SP, Harris SE, Davies G et al (2016) Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol Psychiatry 21:1624–1632. https://doi.org/10.1038/mp.2015.225
Hanson JM, Kinsella JE (1981) Fatty acid content and composition of infant formulas and cereals. J Am Diet Assoc 78:250–255
Hartwig FP, Davies NM, Horta BL et al (2016) Effect modification of FADS2 polymorphisms on the association between breastfeeding and intelligence: protocol for a collaborative meta-analysis. BMJ Open 6:e010067. https://doi.org/10.1136/bmjopen-2015-010067
Hartwig FP, Davies NM, Horta BL et al (2019) Effect modification of FADS2 polymorphisms on the association between breastfeeding and intelligence: results from a collaborative meta-analysis. Int J Epidemiol 48:45–57. https://doi.org/10.1093/ije/dyy273
Health and Social Care Information Centre, IFF Research (2012) Infant Feeding Survey 2010. The Health and Social Care Information Centre
Hill WG, Goddard ME, Visscher PM (2008) Data and Theory Point to Mainly Additive Genetic Variance for Complex Traits. PLOS Genet 4:e1000008. https://doi.org/10.1371/journal.pgen.1000008
Hivert V, Sidorenko J, Rohart F et al (2021) Estimation of non-additive genetic variance in human complex traits from a large sample of unrelated individuals. Am J Hum Genet 108:786–798. https://doi.org/10.1016/j.ajhg.2021.02.014
Horta BL (2015) Loret de Mola C, Victora CG (2015) Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr Oslo nor 104:14–19. https://doi.org/10.1111/apa.13139
Horta BL, de Sousa BA, de Mola CL (2018) Breastfeeding and neurodevelopmental outcomes. Curr Opin Clin Nutr Metab Care 21:174–178. https://doi.org/10.1097/MCO.0000000000000453
Hughes RA, Heron J, Sterne JAC, Tilling K (2019) Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol 48:1294–1304. https://doi.org/10.1093/ije/dyz032
Julkunen H, Cichońska A, Tiainen M, et al (2022) Atlas of plasma nuclear magnetic resonance biomarkers for health and disease in 118,461 individuals from the UK Biobank. 2022.06.13.22276332
Keller MC (2014) Gene × environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry 75:18–24. https://doi.org/10.1016/j.biopsych.2013.09.006
Kent P (2017) Fluid intelligence: a brief history. Appl Neuropsychol Child 6:193–203. https://doi.org/10.1080/21622965.2017.1317480
Kim H-Y, Huang BX, Spector AA (2014) Phosphatidylserine in the brain: metabolism and function. Prog Lipid Res 56:1–18. https://doi.org/10.1016/j.plipres.2014.06.002
Koletzko B, Agostoni C, Carlson S et al (2001) Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. Acta Paediatr 90:460–464. https://doi.org/10.1111/j.1651-2227.2001.tb00452.x
Koletzko B, Lattka E, Zeilinger S et al (2011) Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon longitudinal study of parents and children. Am J Clin Nutr 93:211–219. https://doi.org/10.3945/ajcn.110.006189
Koletzko B, Carlson SE, van Goudoever JB (2015) Should infant formula provide both omega-3 DHA and omega-6 arachidonic acid? Ann Nutr Metab 66:137–138. https://doi.org/10.1159/000377643
Koletzko B, Bergmann K, Brenna JT et al (2020) Should formula for infants provide arachidonic acid along with DHA? A position paper of the European academy of paediatrics and the child health foundation. Am J Clin Nutr 111:10–16. https://doi.org/10.1093/ajcn/nqz252
Kramer MS, Aboud F, Mironova E et al (2008) Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry 65:578–584. https://doi.org/10.1001/archpsyc.65.5.578
Kusunoki T, Morimoto T, Nishikomori R et al (2010) Breastfeeding and the prevalence of allergic diseases in schoolchildren: Does reverse causation matter? Pediatr Allergy Immunol 21:60–66. https://doi.org/10.1111/j.1399-3038.2009.00982.x
Lattka E, Illig T, Koletzko B, Heinrich J (2010) Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr Opin Lipidol 21:64. https://doi.org/10.1097/MOL.0b013e3283327ca8
Lattka E, Klopp N, Demmelmair H et al (2012) Genetic variations in polyunsaturated fatty acid metabolism – implications for child health? Ann Nutr Metab 60:8–17. https://doi.org/10.1159/000337308
Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF (2001) The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 40:1–94. https://doi.org/10.1016/s0163-7827(00)00017-5
Lawn RB, Sallis HM, Taylor AE et al (2019) Schizophrenia risk and reproductive success: a Mendelian randomization study. R Soc Open Sci 6:181049. https://doi.org/10.1098/rsos.181049
Lee JJ, Wedow R, Okbay A et al (2018) Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 50:1112–1121. https://doi.org/10.1038/s41588-018-0147-3
Lepping RJ, Honea RA, Martin LE et al (2019) Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev Psychobiol 61:5–16. https://doi.org/10.1002/dev.21780
Liao K, McCandliss BD, Carlson SE et al (2017) Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev Sci. https://doi.org/10.1111/desc.12455
Lin YH, Llanos A, Mena P et al (2010) Compartmental analyses of 2H5-α-linolenic acid and C-U-eicosapentaenoic acid toward synthesis of plasma labeled 22:6n–3 in newborn term infants. Am J Clin Nutr 92:284–293. https://doi.org/10.3945/ajcn.2009.28779
Lin B, Dai R, Lu L et al (2020) Breastfeeding and atopic dermatitis risk: a systematic review and meta-analysis of prospective cohort studies. Dermatol Basel Switz 236:345–360. https://doi.org/10.1159/000503781
Little J, Higgins JPT, Ioannidis JPA et al (2009) Strengthening the reporting of genetic association studies (STREGA)–an extension of the strobe statement. Genet Epidemiol 33:581–598. https://doi.org/10.1002/gepi.20410
Lodge CJ, Lowe AJ, Dharmage SC (2008) Is reverse causation responsible for the link between duration of breastfeeding and childhood asthma? Am J Respir Crit Care Med 178:994–994. https://doi.org/10.1164/ajrccm.178.9.994a
Lowe AJ, Thien FCK, Stoney RM et al (2008) Associations between fatty acids in colostrum and breast milk and risk of allergic disease. Clin Exp Allergy 38:1745–1751. https://doi.org/10.1111/j.1365-2222.2008.03073.x
Lyall DM, Cullen B, Allerhand M et al (2016) Cognitive test scores in UK Biobank: Data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS ONE 11:e0154222. https://doi.org/10.1371/journal.pone.0154222
Manichaikul A, Mychaleckyj JC, Rich SS et al (2010) Robust relationship inference in genome-wide association studies. Bioinforma Oxf Engl 26:2867–2873. https://doi.org/10.1093/bioinformatics/btq559
Martin NW, Benyamin B, Hansell NK et al (2011) Cognitive function in adolescence: testing for interactions between breast-feeding and FADS2 polymorphisms. J Am Acad Child Adolesc Psychiatry 50:55-62.e4. https://doi.org/10.1016/j.jaac.2010.10.010
Martin J (1978) Infant Feeding 1975: Attitudes and Practice in England and Wales : a Survey Carried Out on Behalf of the Department of Health and Social Security. H.M. Stationery Office
Mathieson I, Day FR, Barban N et al (2023) Genome-wide analysis identifies genetic effects on reproductive success and ongoing natural selection at the FADS locus. Nat Hum Behav 7:790–801. https://doi.org/10.1038/s41562-023-01528-6
Metherel AH, Bazinet RP (2019) Updates to the n-3 polyunsaturated fatty acid biosynthesis pathway: DHA synthesis rates, tetracosahexaenoic acid and (minimal) retroconversion. Prog Lipid Res 76:101008. https://doi.org/10.1016/j.plipres.2019.101008
Michaelsen KF, Lauritzen L, Mortensen EL (2009) Effects of breast-feeding on cognitive function. In: Goldberg G, Prentice A, Prentice A et al (eds) Breast-feeding: early influences on later health. Springer, Dordrecht, pp 199–215
Munafò MR, Tilling K, Taylor AE et al (2018) Collider scope: when selection bias can substantially influence observed associations. Int J Epidemiol 47:226–235. https://doi.org/10.1093/ije/dyx206
Nevins JEH, Donovan SM, Snetselaar L et al (2021) Omega-3 fatty acid dietary supplements consumed during pregnancy and lactation and child neurodevelopment: a systematic review. J Nutr 151:3483–3494. https://doi.org/10.1093/jn/nxab238
Newby D, Winchester L, Sproviero W et al (2021) Associations between brain volumes and cognitive tests with hypertensive burden in UK Biobank. J Alzheimers Dis 84:1373–1389. https://doi.org/10.3233/JAD-210512
Oddy WH, Pal S, Kusel MMH et al (2006) Atopy, eczema and breast milk fatty acids in a high-risk cohort of children followed from birth to 5 yr. Pediatr Allergy Immunol 17:4–10. https://doi.org/10.1111/j.1399-3038.2005.00340.x
Okbay A, Rietveld CA (2015) On improving the credibility of candidate gene studies: a review of candidate gene studies published in Emotion. Emotion 15:531–537. https://doi.org/10.1037/emo0000076
Okbay A, Beauchamp JP, Fontana MA et al (2016) Genome-wide association study identifies 74 loci associated with educational attainment. Nature 533:539–542. https://doi.org/10.1038/nature17671
WHO Collaborative Study on Breast-Feeding, Organization WH (1981) Contemporary patterns of breast-feeding : report on the WHO Collaborative Study on Breast-feeding. World Health Organization
Peters RL, Kay T, McWilliam VL et al (2021) The interplay between eczema and breastfeeding practices may hide breastfeeding’s protective effect on childhood asthma. J Allergy Clin Immunol Pract 9:862-871.e5. https://doi.org/10.1016/j.jaip.2020.09.006
Price AL, Patterson NJ, Plenge RM et al (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38:904–909. https://doi.org/10.1038/ng1847
Purcell S, Chang C (2022) PLINK 2.00a3.7LM AVX2 Intel
Ramsden S, Richardson FM, Josse G et al (2011) Verbal and non-verbal intelligence changes in the teenage brain. Nature 479:113–116. https://doi.org/10.1038/nature10514
Ritchie SJ (2017) Publication bias in a recent meta-analysis on breastfeeding and IQ. Acta Paediatr 106:345–345. https://doi.org/10.1111/apa.13539
Salthouse TA (2009) When does age-related cognitive decline begin? Neurobiol Aging 30:507–514. https://doi.org/10.1016/j.neurobiolaging.2008.09.023
Sauerwald TU, Hachey DL, Jensen CL et al (1996) Effect of dietary alpha-linolenic acid intake on incorporation of docosahexaenoic and arachidonic acids into plasma phospholipids of term infants. Lipids 31(Suppl):S131-135. https://doi.org/10.1007/BF02637064
Savage JE, Jansen PR, Stringer S et al (2018) Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet 50:912–919. https://doi.org/10.1038/s41588-018-0152-6
Schaid DJ, Chen W, Larson NB (2018) From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat Rev Genet 19:491–504. https://doi.org/10.1038/s41576-018-0016-z
Schneider W, Niklas F, Schmiedeler S (2014) Intellectual development from early childhood to early adulthood: The impact of early IQ differences on stability and change over time. Learn Individ Differ 32:156–162. https://doi.org/10.1016/j.lindif.2014.02.001
Scholtz SA, Kerling EH, Shaddy DJ et al (2015) Docosahexaenoic acid (DHA) supplementation in pregnancy differentially modulates arachidonic acid and DHA status across FADS genotypes in pregnancy. Prostaglandins Leukot Essent Fatty Acids 94:29–33. https://doi.org/10.1016/j.plefa.2014.10.008
Simpson DA, Quigley MA, Kurinczuk JJ, Carson C (2019) Twenty-five-year trends in breastfeeding initiation: The effects of sociodemographic changes in Great Britain, 1985–2010. PLoS ONE 14:e0210838. https://doi.org/10.1371/journal.pone.0210838
Singmann P, Rzehak P, Berdel D et al (2010) No association between FADS polymorphisms and atopic diseases in children from the GINI and LISA birth cohorts. Allergy 65:1627–1629. https://doi.org/10.1111/j.1398-9995.2010.02457.x
Smit EN, Fokkema MR, Boersma ER, Muskiet FAJ (2003) Higher erythrocyte 22: 6n–3 and 22: 5n–6, and lower 22: 5n–3 suggest higher Δ-4-desaturation capacity in women of childbearing age. Br J Nutr 89:739–740. https://doi.org/10.1079/BJN2003851
Stamatakis E, Owen KB, Shepherd L et al (2021) Is cohort representativeness passé? Poststratified associations of lifestyle risk factors with mortality in the UK Biobank. Epidemiology 32:179. https://doi.org/10.1097/EDE.0000000000001316
Steer CD, Smith GD, Emmett PM et al (2010) FADS2 polymorphisms modify the effect of breastfeeding on child IQ. PLoS ONE 5:e11570. https://doi.org/10.1371/journal.pone.0011570
Stevens EE, Patrick TE, Pickler R (2009) A history of infant feeding. J Perinat Educ 18:32–39. https://doi.org/10.1624/105812409X426314
Sullivan PF (2007) Spurious genetic associations. Biol Psychiatry 61:1121–1126. https://doi.org/10.1016/j.biopsych.2006.11.010
Sun GY, Simonyi A, Fritsche KL et al (2018) Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids 136:3–13. https://doi.org/10.1016/j.plefa.2017.03.006
Thorley V (2019) Is breastfeeding ‘normal’? Using the right language for breastfeeding. Midwifery 69:39–44. https://doi.org/10.1016/j.midw.2018.10.015
Victora CG, Bahl R, Barros AJD et al (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. The Lancet 387:475–490. https://doi.org/10.1016/S0140-6736(15)01024-7
Walfisch A, Sermer C, Cressman A, Koren G (2013) Breast milk and cognitive development–the role of confounders: a systematic review. BMJ Open 3:e003259. https://doi.org/10.1136/bmjopen-2013-003259
Wang G, Bhatta L, Moen G-H et al (2022) Investigating a potential causal relationship between maternal blood pressure during pregnancy and future offspring cardiometabolic health. Hypertens Dallas Tex 79:170–177. https://doi.org/10.1161/HYPERTENSIONAHA.121.17701
Warrington NM, Hwang L-D, Nivard MG, Evans DM (2021) Estimating direct and indirect genetic effects on offspring phenotypes using genome-wide summary results data. Nat Commun 12:5420. https://doi.org/10.1038/s41467-021-25723-z
Whitaker S (2008) The stability of IQ in people with low intellectual ability: an analysis of the literature. Intellect Dev Disabil 46:120–128. https://doi.org/10.1352/0047-6765(2008)46[120:TSOIIP]2.0.CO;2
Whitcomb BW, Naimi AI (2020) Things don’t always go as expected: the example of nondifferential misclassification of exposure—bias and error. Am J Epidemiol 189:365–368. https://doi.org/10.1093/aje/kwaa020
Wijga AH, van Houwelingen AC, Kerkhof M et al (2006) Breast milk fatty acids and allergic disease in preschool children: The prevention and incidence of asthma and mite allergy birth cohort study. J Allergy Clin Immunol 117:440–447. https://doi.org/10.1016/j.jaci.2005.10.022
World Health Organization (2001) The optimal duration of exclusive breastfeeding: a systematic review
World Health Organization (2003) Global strategy for infant and young child feeding. World Health Organization
Xie L, Innis SM (2008) Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr 138:2222–2228. https://doi.org/10.3945/jn.108.096156
Yang YW, Tsai CL, Lu CY (2009) Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol 161:373–383. https://doi.org/10.1111/j.1365-2133.2009.09049.x
Yeates AJ, Love TM, Engström K et al (2015) Genetic variation in FADS genes is associated with maternal long-chain PUFA status but not with cognitive development of infants in a high fish-eating observational study. Prostaglandins Leukot Essent Fatty Acids 102–103:13–20. https://doi.org/10.1016/j.plefa.2015.08.004
Yzerbyt VY, Muller D, Judd CM (2004) Adjusting researchers’ approach to adjustment: On the use of covariates when testing interactions. J Exp Soc Psychol 40:424–431. https://doi.org/10.1016/j.jesp.2003.10.001
Zhang Z, Wang Y, Yang X et al (2022) Human Milk lipid profiles around the world: a systematic review and meta-analysis. Adv Nutr 13:2519–2536. https://doi.org/10.1093/advances/nmac097
Zondervan KT, Cardon LR (2007) Designing candidate gene and genome-wide case-control association studies. Nat Protoc 2:2492–2501. https://doi.org/10.1038/nprot.2007.366
Acknowledgements
We would like to thank the UK Biobank participants, interviewers, nurses, and staff that made this study possible. We wish to acknowledge The University of Queensland's Research Computing Centre (RCC) for its support in this research.
Funding
This work was supported by an NHMRC Ideas Grant [APP1157714]. UK Biobank is generously supported by its founding funders the Wellcome Trust and UK Medical Research Council, as well as the British Heart Foundation, Cancer Research UK, Department of Health, Northwest Regional Development Agency and Scottish Government. D.M.E. is supported by an NHMRC Leadership Fellowship [APP2017942]. N.M.W is supported by an NHMRC Emerging Leadership Fellowship [APP2008723]. G.H. and G.D.S. were supported by the UK Medical Research Council [MC_UU_00032/01]. The analyses described in this manuscript were conducted under the UK Biobank application 53641.
Author information
Authors and Affiliations
Contributions
GC, NW, GH, GDS, and DME designed the research; GC analysed the data; GC, NW, and DME wrote the paper. GW provided help on the analysis for the blood pressure phenotypes. DME had primary responsibility for final content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Edited by Brad Verhulst.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Centorame, G., Warrington, N.M., Hemani, G. et al. No Evidence of Interaction Between FADS2 Genotype and Breastfeeding on Cognitive or Other Traits in the UK Biobank. Behav Genet 55, 86–102 (2025). https://doi.org/10.1007/s10519-024-10210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-024-10210-0