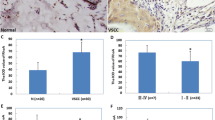
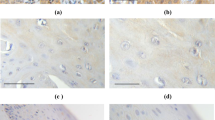
Lichen sclerosus of the vulva is a common, but poorly studied disease. We assessed the level of transcriptional activity of APAF1, BAX, BCL2, BIRC5, CCND1, DAPK1, MCL1, and MYC genes encoding products that control apoptosis in the samples of tissues affected by vulvar lichen sclerosus and adjacent control tissues (n=24). Analysis of transcriptional activity was performed by real-time PCR using specific primers and SYBR Green intercalating dye. After the total group was divided by the presence of the concomitant gynecological diseases, a significant increase in the transcriptional activity of the CCND1 gene was revealed in patients with concomitant uterine fibroids. This may indicate the possible role of the activation of mitosis during tumor initiation.
Similar content being viewed by others
References
Urmancheeva AF. Epidemiology of vulval cancer. Risk factors and prognosis. Prakt. Onkol. 2006;7(4):189-196. Russian.
Angeloni D, ter Elst A, Wei MH, van der Veen AY, Braga EA, Klimov EA, Timmer T, Korobeinikova L, Lerman MI, Buys CH. Analysis of a new homozygous deletion in the tumor suppressor region at 3p12.3 reveals two novel intronic noncoding RNA genes. Genes Chromosomes Cancer. 2006;45(7):676-691. doi: https://doi.org/10.1002/gcc.20332
Ben-Hur H, Ashkenazi M, Huszar M, Gurevich P, Zusman I. Lymphoid elements and apoptosis-related proteins (Fas, Fas ligand, p53 and bcl-2) in lichen sclerosus and carcinoma of the vulva. Eur. J. Gynaecol. Oncol. 2001;22(2):104-109.
Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. Experimental transmission of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J. Exp. Med. 1999;190(12):1857-1868. doi: https://doi.org/10.1084/jem.190.12.1857
Hillemanns P, Wang X. Integration of HPV-16 and HPV-18 DNA in vulvar intraepithelial neoplasia. Gynecol. Oncol. 2006;100(2):276-282. doi: https://doi.org/10.1016/j.ygyno.2005.10.003
Kaczanowski S. Apoptosis: its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016;13(3):031001. doi: https://doi.org/10.1088/1478-3975/13/3/031001
Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37(7):8471-8486. doi: https://doi.org/10.1007/s13277-016-5035-9
Kontos CK, Fendri A, Khabir A, Mokdad-Gargouri R, Scorilas A. Quantitative expression analysis and prognostic significance of the BCL2-associated X gene in nasopharyngeal carcinoma: a retrospective cohort study. BMC Cancer. 2013;13:293. doi: https://doi.org/10.1186/1471-2407-13-293
Luo L, Zhang T, Liu H, Lv T, Yuan D, Yao Y, Lv Y, Song Y. MiR-101 and Mcl-1 in non-small-cell lung cancer: expression profile and clinical significance. Med. Oncol. 2012;29(3):1681-1686. doi: https://doi.org/10.1007/s12032-011-0085-8
Montalto FI, De Amicis F. Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells. 2020;9(12):2648. doi: https://doi.org/10.3390/cells9122648
Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. (Berl). 2016;94(12):1313- 1326. doi: https://doi.org/10.1007/s00109-016-1475-3
Singh N, Ghatage P. Etiology, clinical features, and diagnosis of vulvar lichen sclerosus: a scoping review. Obstet. Gynecol. Int. 2020;2020):7480754. doi: 10.1155/2020/7480754
Wang J, Jia R, Zhang Y, Xu X, Song X, Zhou Y, Zhang H, Ge S, Fan X. The role of Bax and Bcl-2 in gemcitabine-mediated cytotoxicity in uveal melanoma cells. Tumour Biol. 2014;35(2):1169-1175. doi: https://doi.org/10.1007/s13277-013-1156-6
Wang LQ, Kwong YL, Wong KF, Kho CS, Jin DY, Tse E, Rosèn A, Chim CS. Epigenetic inactivation of mir-34b/c in addition to mir-34a and DAPK1 in chronic lymphocytic leukemia. J. Transl. Med. 2014;12:52. doi: https://doi.org/10.1186/1479-5876-12-52
Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30(1):87. doi: https://doi.org/10.1186/1756-9966-30-87
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 172, No. 12, pp. 735-739, December, 2021
Rights and permissions
About this article
Cite this article
Klimov, E.A., Sobolev, V.V., Batashkov, N.A. et al. Transcriptional Activity of Some Genes Involved in Apoptosis in Patients with Vulvar Lichen Sclerosus. Bull Exp Biol Med 172, 734–737 (2022). https://doi.org/10.1007/s10517-022-05467-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-022-05467-6