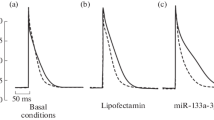
Cardiac-specific microRNA miR-133a-3p modulates adrenergic signaling. Adrenergic receptors and their intracellular pathways are the key players in proarrhythmic ectopy derived from the myocardial sleeves of the pulmonary veins. We studied the effect of miR-133a-3p on ectopy induced by norepinephrine in myocardial tissue of rat pulmonary veins. Using microelectrode technique, we revealed facilitation of proarrhythmic pattern of spontaneous bursts of action potentials induced by norepinephrine in tissue preparations of the pulmonary veins isolated from rats in 24 h after injection of a transfection mixture containing miR-133a-3p (1 mg/kg) in vivo. According to ELISA data, the cAMP level in the pulmonary vein myocardium of rats receiving miR-133a-3p was 2-fold higher than in control animals. Bioinformatic analysis showed that mRNA of protein phosphatases and some phosphodiesterases are most probable targets of miR-133a-3p. The proarrhythmic effect of miR-133a-3p can be related to inhibition of the expression of phosphodiesterases accompanied by cAMP accumulation and increased intracellular β-adrenergic signaling.
Similar content being viewed by others
References
Andrés MD. Therapeutic potential for miR-133a/b to prevent zinc finger homeobox 3 loss of function-dependent atrial fibrillation. Acta Physiol. (Oxf). 2019;227(3):e13372. https://doi.org/10.1111/apha.13372.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215-233. https://doi.org/10.1016/j.cell.2009.01.002
Belevych AE, Sansom SE, Terentyeva R, Ho HT, Nishijima Y, Martin MM, Jindal HK, Rochira JA, Kunitomo Y, Abdellatif M, Carnes CA, Elton TS, Györke S, Terentyev D. MicroRNA-1 and -133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex. PLoS One. 2011;6(12):e28324. https://doi.org/10.1371/journal.pone.0028324
Castaldi A, Zaglia T, Di Mauro V, Carullo P, Viggiani G, Borile G, Di Stefano B, Schiattarella GG, Gualazzi MG, Elia L, Stirparo GG, Colorito ML, Pironti G, Kunderfranco P, Esposito G, Bang ML, Mongillo M, Condorelli G, Catalucci D. MicroRNA-133 modulates the β1-adrenergic receptor transduction cascade. Circ. Res. 2014;115(2):273-283. https://doi.org/10.1161/CIRCRESAHA.115.303252
Condorelli G, Latronico M. V, Cavarretta E. microRNAs in cardiovascular diseases: current knowledge and the road ahead. J. Am. Coll. Cardiol. 2014;63(21):2177-2187. https://doi.org/10.1016/j.jacc.2014.01.050
Deiuliis JA. MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. (Lond). 2016;40(1):88-101. https://doi.org/10.1038/ijo.2015.170
D’Souza A, Pearman CM, Wang Y, Nakao S, Logantha SJRJ, Cox C, Bennett H, Zhang Y, Johnsen AB, Linscheid N, Poulsen PC, Elliott J, Coulson J, McPhee J, Robertson A, da Costa Martins PA, Kitmitto A, Wisløff U, Cartwright EJ, Monfredi O, Lundby A, Dobrzynski H, Oceandy D, Morris GM, Boyett MR. Targeting miR-423-5p reverses exercise training-induced HCN4 channel remodeling and sinus bradycardia. Circ. Res. 2017;121(9):1058-1068. https://doi.org/10.1161/CIRCRESAHA.117.311607
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998;339(10):659-666. https://doi.org/10.1056/NEJM199809033391003
Karimova VM, Pustovit KB, Abramochkin DV, Kuz’min VS. Effect of purine co-transmitters on automatic activity caused by norepinephrine in myocardial sleeves of pulmonary veins. Bull. Exp. Biol. Med. 2017;162(5):589-593. https://doi.org/10.1007/s10517-017-3664-7
Kuzmin VS, Ivanova AD, Filatova TS, Pustovit KB, Kobylina AA, Atkinson AJ, Petkova M, Voronkov YI, Abramochkin DV, Dobrzynski H. Micro-RNA 133a-3p induces repolarization abnormalities in atrial myocardium and modulates ventricular electrophysiology affecting I Ca,L and Ito currents. Eur. J. Pharmacol. 2021;908:174369. https://doi.org/10.1016/j.ejphar.2021.174369
Kuzmin VS, Ivanova AD, Potekhina VM, Samoilova DV, Ushenin KS, Shvetsova AA, Petrov AM. The susceptibility of the rat pulmonary and caval vein myocardium to the catecholamineinduced ectopy changes oppositely in postnatal development. J. Physiol. 2021;599(11):2803-2821. https://doi.org/10.1113/JP280485
Mikhailov AT, Torrado M. Interplay between cardiac transcription factors and non-coding RNAs in predisposing to atrial fibrillation. J. Mol. Med. (Berl). 2018;96(7):601-610. https://doi.org/10.1007/s00109-018-1647-4
Petkova M, Atkinson AJ, Yanni J, Stuart L, Aminu AJ, Ivanova AD, Pustovit KB, Geragthy C, Feather A, Li N, Zhang Y, Oceandy D, Perde F, Molenaar P, D’Souza A, Fedorov VV, Dobrzynski H. Identification of key small non-coding microRNAs controlling pacemaker mechanisms in the human sinus node. J. Am. Heart Assoc. 2020;9(20):e016590. https://doi.org/10.1161/JAHA.120.016590
Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2007;42(6):1137-1141. https://doi.org/10.1016/j.yjmcc.2007.04.004
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl Acad. Sci. USA. 2008;105(35):13027-13032. https://doi.org/10.1073/pnas.0805038105
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 172, No. 12, pp. 664-668, December, 2021
Rights and permissions
About this article
Cite this article
Kuz’min, V.S., Kobylina, A.A., Pustovit, K.B. et al. MicroRNA miR-133a-3p Facilitates Adrenergic Proarrhythmic Ectopy in Rat Pulmonary Vein Myocardium by Increasing cAMP Content. Bull Exp Biol Med 172, 671–675 (2022). https://doi.org/10.1007/s10517-022-05454-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-022-05454-x