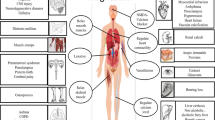
We studied chronic toxicity of a few release-active preparations: Dietressa (release-active preparation of affinity-purified antibodies to type 1 cannabinoid receptor), Divasa (releaseactive preparation containing a combination of affinity-purified antibodies to brain-specific S-100 protein and endothelial NO-synthase), Cardostin (release-active preparation containing a combination of affinity-purified antibodies to C-terminal fragment of angiotensin II type 1 receptor and endothelial NO-synthase), and Bation (release-active preparation containing a combination of affinity-purified antibodies to IFN-γ and CD4). We evaluated not only side and toxic effects, but also the relationships between these effects and pharmacological activities of the preparations. The data of preclinical toxicological studies of the release-active preparations can be used for prediction of their pharmacological activity.
Similar content being viewed by others
References
T. A. Voronina, G. M. Molodavkin, E. S. Zhavbert, S. A. Tarasov, I. A. Kheyfets, J. L. Dugina, S. A. Sergeeva, and O. I. Epstein, Anxiolytic and antidepressant characteristics of impaza. Bull. Exp. Biol. Med., 145, No. 6, 735-737 (2008).
S. N. Golikov, I. V. Sanovitskii, and L. A. Tiunov, General Modes of Toxicity [in Russian], Leningrad (1986).
N. N. Gur’yanova, Yu. L. Dugina, E. S. Zhavbert, and O. I. Epstein, Prospects of using Dietressa, a novel preparation containing antibodies to type 1 cannabinoid receptor, in the therapy of obesity. Vestn. Mezhdunarod. Akad. Nauk. Russkaya Sektsiya, No. 1, 34-38 (2012).
E. S. Zhavbert, Yu. L. Dugina, and O. I. Epstein, Immunotropic Properties of Anaferon and Anaferon Pediatric. Antibiotiki Khimioterapiya, 58, Nos. 5-6, 17-23 (2013).
G. V. Karpova, T. I. Fomina, T. V. Vetoshkina, T. Yu. Dubskaya, O. L. Voronova, E. A. Timina, E. V. Abramova, L. A. Ermolaeva, and A. V. Perova, Preclinical Studie of General Toxic Properties of Preparations Containing Ultralow Doses of Antibodies to Endogenous Regulators. Bull. Exp. Biol. Med., 148, No. 3, 543-546 (2009).
Manual on Experimental (Preclinical) Study of New Pharmacological Substances [in Russian], Ed. R. U. Khabriev, Moscow (2005).
I. A. Kheyfets, L. I. Bugaeva, T. M. Vorob’eva, J. L. Dugina, S. A. Lebedeva, V. I. Petrov, S. A. Sergeeva, and O. I. Epstein, Experimental Study of the Effects of Dietressa, a New Weight-Reducing Drug. Bull. Exp. Biol. Med., 152, No. 3, 321-324 (2012).
P. D. Shabanov, I. B. Bazilenko, and D. A. Venkov, Nonben zodiazepine tranquilizers. Pharmacology of Tenoten as an Anxiolytic, Stress Protector and Adaptogen. Psikhofarmakol. Biol. Narkol., 8, No. 1-2-1, 2264-2270 (2008).
O. I. Epstein, The phenomenon of release activity and the hypothesis of “spatial” homeostasis. Uspekhi Fiziol. Nauk, 44, No. 3, 54-76 (2013).
F. R. Brennan, L. D. Morton, S. Spindeldreher, A. Kiessling, R. Allenspach, A. Hey, P. Y. Muller, W. Frings, and J. Sims, Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs, 2, No. 3, 233-255 (2010).
Bugaeva L.I., Verovskii V.E., Iezhitsa I.N., Spasov A.A. Investigation of the acute toxicity of bromantan. Exp. Klin. Farmakol. 2000. 63, No. 1, 57-61.
L. Murray, F. Daly, M. Little, and M. Cadogan, Toxicology Handbook, 2nd ed., Churchill Livingstone (2010).
C. J. Trivedi, R. Balaraman, J. B. Majithiya, and S. B. Bothara, Effect of atorvastatin treatment on isoproterenol-induced myocardial infarction in rats. Pharmacology, 77, No. 1, 25-32 (2006).
Predictive Toxicology in Drug Safety, Eds. J. J. Xu and L. Urban, Cambridge (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 161, No. 2, pp. 212-217, February, 2016
Rights and permissions
About this article
Cite this article
Zhavbert, E.S., Surkova, E.I., Yakovleva, N.N. et al. Preclinical Toxicological Study of Release-Active Preparations for Prediction of Their Pharmacological Activity and Side Effects. Bull Exp Biol Med 161, 252–256 (2016). https://doi.org/10.1007/s10517-016-3389-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-016-3389-z