Abstract
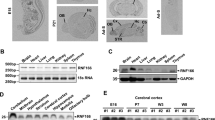
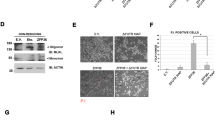
The HIPPI (HIP-1 protein interactor) protein is a multifunctional protein that is involved in the regulation of apoptosis. The interaction partners of HIPPI include HIP-1 (Huntingtin-interacting protein-1), Apoptin, Homer1c, Rybp/DEDAF, and BAR (bifunctional apoptosis regulator). In search for other binding partners of HIPPI, we performed a yeast two hybrid screen and identified BLOC1S2 (Biogenesis of lysosome-related organelles complex-1 subunit 2) as a novel HIPPI-interacting protein. In co-immunoprecipitation assays, BLOC1S2 specifically associates with HIPPI, but not with HIP-1. To study the expression of BLOC1S2 on the protein level, we generated a mouse monoclonal antibody specific for BLOC1S2 and a multiple tissue array comprising 70 normal and cancer tissue samples of diverse origin. BLOC1S2 protein is widely expressed in normal tissue as well as in malignant tumors with a tendency towards lower expression levels in certain subtypes of tumors. On the subcellular level, BLOC1S2 is expressed in an organellar-like pattern and co-localizes with mitochondria. Over-expression of BLOC1S2 in the presence or absence of HIPPI does not induce apoptosis. However, BLOC1S2 and HIPPI sensitize NCH89 glioblastoma cells to the pro-apoptotic actions of staurosporine and the death ligand TRAIL by enhancing caspase activation, cytochrome c release, and disruption of the mitochondrial membrane potential. Given its interaction with HIPPI and its pro-apoptotic activity, BLOC1S2 might play an important functional role in cancer and neurodegenerative diseases.







Similar content being viewed by others
References
Gervais FG, Singaraja R, Xanthoudakis S et al (2002) Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat Cell Biol 4:95–105
Wanker EE (2002) Hip1 and Hippi participate in a novel cell death-signaling pathway. Dev Cell 2:126–128
Cheng CM, Huang SP, Chang YF, Chung WY, Yuo CY (2003) The viral death protein Apoptin interacts with Hippi, the protein interactor of Huntingtin-interacting protein 1. Biochem Biophys Res Commun 305:359–364
Sakamoto K, Yoshida S, Ikegami K et al (2007) Homer1c interacts with Hippi and protects striatal neurons from apoptosis. Biochem Biophys Res Commun 352:1–5
Stanton SE, Blanck JK, Locker J, Schreiber-Agus N (2007) Rybp interacts with Hippi and enhances Hippi-mediated apoptosis. Apoptosis 12:2197–2206
Roth W, Kermer P, Krajewska M et al (2003) Bifunctional apoptosis inhibitor (BAR) protects neurons from diverse cell death pathways. Cell Death Differ 10:1178–1187
Majumder P, Chattopadhyay B, Sukanya S et al (2007) Interaction of HIPPI with putative promoter sequence of caspase-1 in vitro and in vivo. Biochem Biophys Res Commun 353:80–85
Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC (2003) IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J Biol Chem 278:34211–34218
Haycraft CJ, Schafer JC, Zhang Q, Taulman PD, Yoder BK (2003) Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Exp Cell Res 284:251–263
Houde C, Dickinson RJ, Houtzager VM et al (2006) Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol 300:523–533
Matsuzawa S, Takayama S, Froesch BA, Zapata JM, Reed JC (1998) p53-inducible human homologue of Drosophila seven in absentia (Siah) inhibits cell growth: suppression by BAG-1. The EMBO J 17:2736–2747
Matsuzawa SI, Reed JC (2001) Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell 7:915–926
Karcher S, Steiner HH, Ahmadi R et al (2006) Different angiogenic phenotypes in primary and secondary glioblastomas. Int J Cancer 118:2182–2189
Schultze K, Bock B, Eckert A et al (2006) Troglitazone sensitizes tumor cells to TRAIL-induced apoptosis via down-regulation of FLIP and Survivin. Apoptosis 11:1503–1512
Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Eckert A, Bock BC, Tagscherer KE et al (2007) The PEA-15/PED protein protects glioblastoma cells from glucose deprivation-induced apoptosis via the ERK/MAP kinase pathway. Oncogene [Epub ahead of print]
Starcevic M, Dell’Angelica EC (2004) Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J Biol Chem 279:28393–28401
Wang Z, Wei H, Yu Y et al (2004) Characterization of Ceap-11 and Ceap-16, two novel splicing-variant-proteins, associated with centrosome, microtubule aggregation and cell proliferation. J Mol Biol 343:71–82
Dell’Angelica EC (2004) The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol 16:458–464
Di Pietro SM, Falcon-Perez JM, Dell’Angelica EC (2004) Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic 5:276–283
Falcon-Perez JM, Romero-Calderon R, Brooks ES, Krantz DE, Dell’Angelica EC (2007) The Drosophila pigmentation gene pink (p) encodes a homologue of human Hermansky-Pudlak syndrome 5 (HPS5). Traffic 8:154–168
Huizing M, Parkes JM, Helip-Wooley A, White JG, Gahl WA (2007) Platelet alpha granules in BLOC-2 and BLOC-3 subtypes of Hermansky-Pudlak syndrome. Platelets 18:150–157
Felten A, Leister P, Burgdorf S, Uhlmann L, Scheidtmann KH (2007) Characterization of rat BLOS2/Ceap, a putative yeast She3 homolog, as interaction partner of apoptosis antagonizing transcription factor/Che-1. Biol Chem 388:569–582
Majumder P, Chattopadhyay B, Mazumder A, Das P, Bhattacharyya NP (2006) Induction of apoptosis in cells expressing exogenous Hippi, a molecular partner of huntingtin-interacting protein Hip1. Neurobiol Dis 22:242–256
Scaffidi C, Fulda S, Srinivasan A et al (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687
Acknowledgments
This work was supported by a grant from the Deutsche Krebshilfe to W.R. (German Cancer Aid, Max Eder Program), a fellowship from the Heinrich F.C. Behr Foundation to G.G., the Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg, and grants from the National Institutes of Health, CA69381 and AG15393, to J.C.R. We thank Sarah Messnard for expert technical assistance, Christel Herold-Mende for providing NCH89 glioblastoma cells, and Frank Stenner-Liewen for generation of the pcDNA3-FLAG-HIPPI plasmid.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gdynia, G., Lehmann-Koch, J., Sieber, S. et al. BLOC1S2 interacts with the HIPPI protein and sensitizes NCH89 glioblastoma cells to apoptosis. Apoptosis 13, 437–447 (2008). https://doi.org/10.1007/s10495-007-0176-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0176-3