Abstract
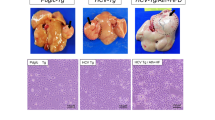
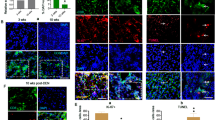
Patients with mutations in the death receptor CD95 (Fas/APO-1) frequently develop B-cell lymphoma. However, solid tumors have not been found in the context of defective CD95. This could be due to the fatal autoimmune proliferative disease that develops in the absence of functional CD95 or to a difference in CD95 signaling in lymphoid versus nonlymphoid tissues. To test this we reconstituted mice that harbor a point mutation in the death domain of CD95 (lprcg mice), either in one or in both alleles, with bone marrow from wild-type (wt) mice. After a year one third of the lprcg/lprcg mice developed spontaneous hepatic neoplasms. In contrast only one of the wt/lprcg mice and none of the wt mice developed liver cancer. The agonistic anti-CD95 antibody Jo2 induced massive apoptosis in the liver of wt mice but not in the livers of either wt/lprcg or lprcg/lprcg mice. The susceptibility of lprcg/lprcg mice to liver cancer cannot solely be due to impaired CD95 mediated apoptosis because there was no clear correlation between apoptosis resistance and tumor formation. A gene chip analysis identified genes selectively upregulated in the liver of wt and wt/lprcg mice which may protect these mice from developing liver cancer. Our data represent the first case of CD95 protecting from developing a solid cancer.




Similar content being viewed by others
Abbreviations
- ADA:
-
Anti-DNA antibodies
- BM:
-
Bone marrow transplantation
- Lpr:
-
Lymphoproliferation
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
References
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10:26–35
Lynch DH et al (1994) The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity 1:131–136
Ramsdell F et al (1994) Gld/gld mice are unable to express a functional ligand for Fas. Eur J Immunol 24:928–933
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317
Kimura M, Matsuzawa A (1994) Autoimmunity in mice bearing lprcg: a novel mutant gene. Int Rev Immunol 11:193–210
Rieux-Laucat F (2006) Inherited and acquired death receptor defects in human Autoimmune Lymphoproliferative Syndrome. Curr Dir Autoimmun 9:18–36
Wajant H, Pfizenmaier K, Scheurich P (2003) Non-apoptotic Fas signaling. Cytokine Growth Factor Rev 14:53–66
Peter ME et al (2007) The CD95 receptor: Apoptosis revisited. Cell 129:447–450
Barnhart BC et al (2004) CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. Embo J 23:3175–3185
Peter ME, Legembre P, Barnhart BC (2005) Does CD95 have tumor promoting activities? Biochim Biophys Acta 1755:25–36
Legembre P et al (2004) Induction of apoptosis and activation of NF-kappaB by CD95 require different signalling thresholds. EMBO Rep 5:1084–1089
Li C, Wong WH (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98:31–36
Mariani SM, Matiba B, Armandola EA, Krammer PH (1994) The APO-1/Fas (CD95) receptor is expressed in homozygous MRL/lpr mice. Eur J Immunol 24:3119–3123
Adachi M et al (1995) Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet 11:294–300
Hao Z, Hampel B, Yagita H, Rajewsky K (2004) T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med 199:1355–1365
Marski M, Kandula S, Turner JR, Abraham C (2005) CD18 is required for optimal development and function of CD4 + CD25 + T regulatory cells. J Immunol 175:7889–7897
Andervont HB (1950) Studies on the occurrence of spontaneous hepatomas in mice of strains C3H and CBA. J Natl Cancer Inst 11:581–592
Desbarats J, Newell MK (2000) Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med 6:920–923
Ogasawara J et al (1993) Lethal effect of the anti-Fas antibody in mice. Nature 364:806–809
Farinati F et al (1996) Hepatocyte proliferative activity in chronic liver damage as assessed by the monoclonal antibody MIB1 Ki67 in archival material: the role of etiology, disease activity, iron, and lipid peroxidation. Hepatology 23:1468–1475
Asefa B et al (2006) p205, a potential tumor suppressor, inhibits cell proliferation via multiple pathways of cell cycle regulation. FEBS Lett 580:1205–1214
Nagata S (1999) Fas ligand-induced apoptosis. Annu Rev Genet 33:29–55
Muschen M, Warskulat U, Beckmann MW (2000) Defining CD95 as a tumor suppressor gene. J Mol Med 78:312–325
Martinez-Chantar ML et al (2002) Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. Faseb J 16:1292–1294
Takahashi Y et al (2002) Enhanced spontaneous and aflatoxin-induced liver tumorigenesis in xeroderma pigmentosum group A gene-deficient mice. Carcinogenesis 23:627–633
Straus SE et al (2001) The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood 98:194–200
Lin A, Karin M (2003) NF-κB in cancer: a marked target. Semin Cancer Biol 13:107–114
Pikarsky E et al (2004) NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431:461–466
Maeda S, Kamata H, Luo JL, Leffert H, Karin M (2005) IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121:977–990
Sakurai T, Maeda S, Chang L, Karin M (2006) Loss of hepatic NF-κ B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA 103:10544–10551
Luedde T et al (2007) Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 11:119–132
Faouzi S et al (2001) Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-κ B-independent, caspase-3-dependent pathway. J Biol Chem 276:49077–49082
Ryschich E et al (2006) Molecular fingerprinting and autocrine growth regulation of endothelial cells in a murine model of hepatocellular carcinoma. Cancer Res 66:198–211
Shi GP et al (1999) Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 10:197–206
Nakagawa TY et al (1999) Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity 10:207–217
Becker FF (1981) Inhibition of spontaneous hepatocarcinogenesis in C3H/HeN mice by transplanted hepatocellular carcinomas. Cancer Res 41:3320–3323
Irie M et al (2004) Inhibition of spontaneous development of liver tumors by inoculation with dendritic cells loaded with hepatocellular carcinoma cells in C3H/HeNCRJ mice. Int J Cancer 111:238–245
Booker JK, Reap EA, Cohen PL (1998) Expression and function of Fas on cells damaged by gamma-irradiation in B6 and B6/lpr mice. J Immunol 161:4536–4541
Apostolou I, Hao Z, Rajewsky K, von Boehmer H (2003) Effective destruction of Fas-deficient insulin-producing beta cells in type 1 diabetes. J Exp Med 198:1103–1106
Yasuda T et al (2000) Immunological characterization of C3H mice congenic for Fas(lprcg), C3h/HeJ-Fas(lprcg)/Fas(lprcg). Lab Anim 34:46–55
Desbarats J et al (2003) Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol 5:118–125
Acknowledgements
We like to thank Terry Lee for performing histology, Xinmin Li for performing the gene array analysis and Nathan Little for help with the AOM/DSS model. We are grateful to Dr. Marisa Alegre for help with the FACS analysis and ELISA used in the study, to Dr. Kazutoshi Sayama for providing the lprcg mice and to Dr. Greg Gores for helpful discussions. The authors have no conflicting financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Park, SM., Rajapaksha, T.W., Zhang, M. et al. CD95 signaling deficient mice with a wild-type hematopoietic system are prone to hepatic neoplasia. Apoptosis 13, 41–51 (2008). https://doi.org/10.1007/s10495-007-0149-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0149-6